Estimate the size of fluorine (liquid) molecule, given its atomic mass 19.01u and density 1.14×103kgm−3. Solution in Kannada
Volume of a single molecule =mass of each moleculedensity
i.e. 43πR3=12.01×1.66×10−272.22×103,1μ=1.66×10−27kg
i.e. R3=3×12.01×1.66×10−302.22×4×3.142=2.144×10−30.
i.e. radius of each molecule R=(2.144)13×10−10m=1.289×10−10m.
1.289Å
Similar Questions
Estimate the average mass density of a sodium atom assuming its size to be about 2.5Å. (Use the known values of Avagardo's number and the atomic mass of sodium). Compare it with the mass density of sodium in its crystalline phase : 970 kg m^(-3) . Are the two densities of the same order of magnitude ? If so, why ?
View SolutionEstimate the volume of a water molecule using the data of one molecule( density of water = 1000kg/m3) What is its approximate size?
View SolutionEstimate the average mass density of a sodium atom assuming its size to be about 2.5Å. (Use the known values of Avagardo's number and the atomic mass of sodium). Compare it with the mass density of sodium in its crystalline phase : 970kgm−3. Are the two densities of the same order of magnitude ? If so, why ?
View SolutionEstimate the average mass density of a sodium atom assuming its size to be about 2.5Å. (Use the known values of Avagardo's number and the atomic mass of sodium). Compare it with the mass density of sodium in its crystalline phase : 970kgm−3. Are the two densities of the same order of magnitude ? If so, why ?
View SolutionFind the radius of atom:
Edge length = 405pm, density = 2.7×103kgm−3, atomic mass = 2.7×10−2kg
View SolutionA metallic sphere floats in immiscible mixture of water (density 10^(3) kgm^(-3)) and a liquid (density 8xx10^(3) kgm^(-3)) such that its (2//3) part is in water and (1//3) part in the liquid. The density of the metal is
View SolutionThe density of gold is 19.6gcm−3. Its density in SI system is ________ kgm−3
View SolutionGiven below are densities of some solids and liquids. Give rough estimates of the size of their atoms :
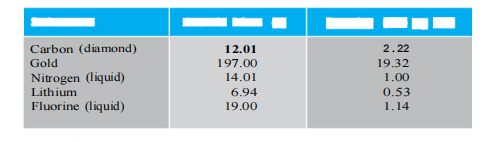
View Solution

