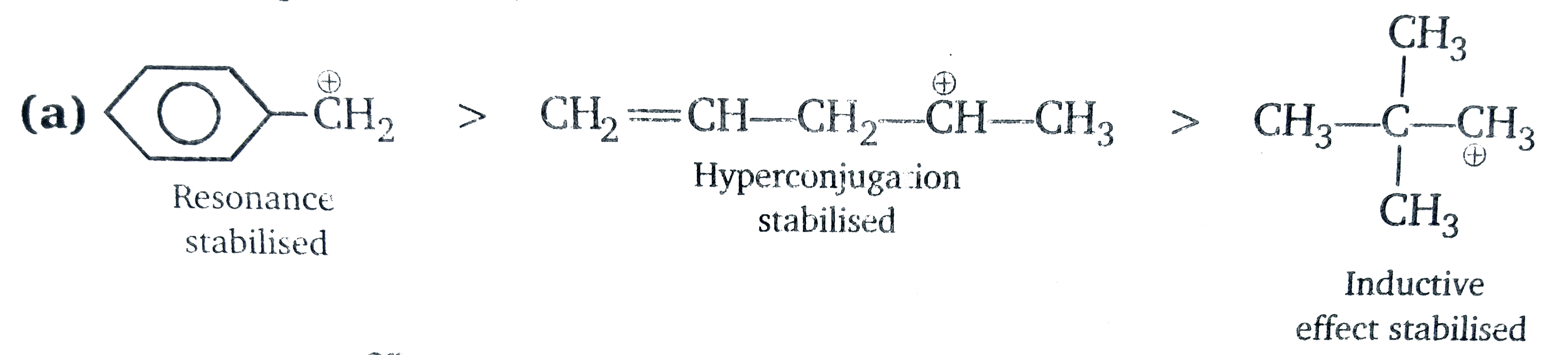Which of the following options in the correct order of relative stabilities of cations I,II and III as written below (most stable first)
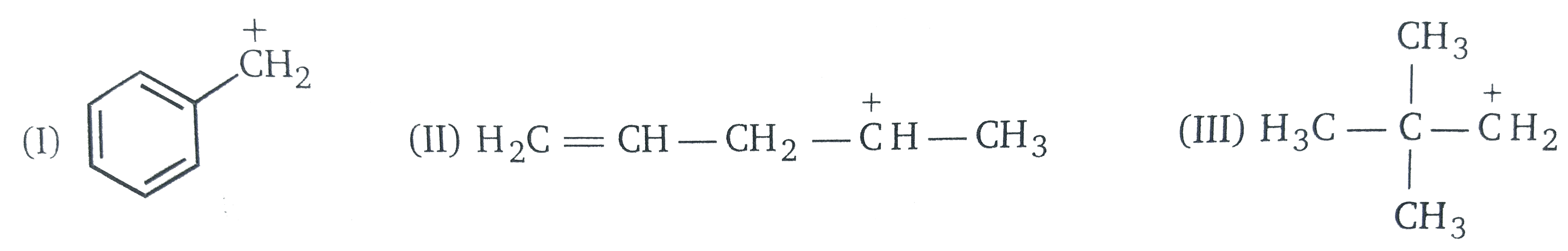
H2C=CH−CH2−+CH−CH3
H3C−CH3∣C∣CH3−+CH2
A
I>II>III
B
II>III>I
C
III>I>II
D
I>III>II
Video Solution
Text Solution
The correct Answer is:A
|
Similar Questions
Knowledge Check
CH3−CH3∣C∣CH3−CH=CH−CH3H−Cl−−−→ ?
ACH3−CH3∣C∣CH3−CH2−CH∣Cl−CH3BCH3−Cl∣C∣CH3−CH−CH2∣CH3−CH3CCH3−CH3∣C∣CH3−C∣ClH=CH2−CH3DCH3−Cl∣C∣CH3−CH2−CH∣CH3−CH3CH3−O∣∣C−CH2−CH3∣C∣CH3−CHOKOH,H2O−−−−−−→(A), product A is
A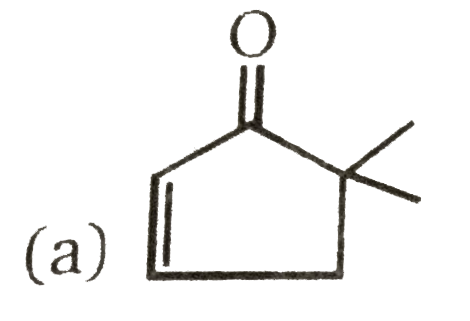 B
B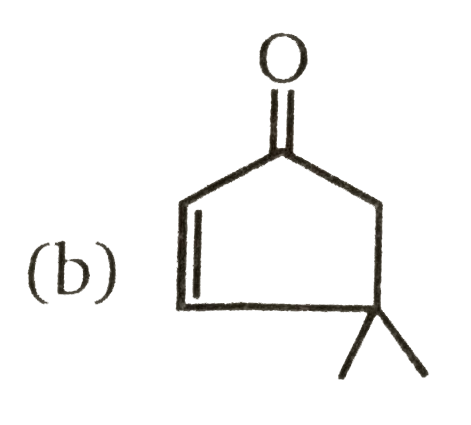 C
C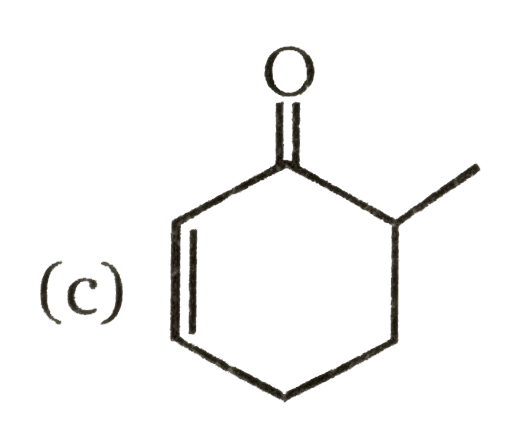 D
D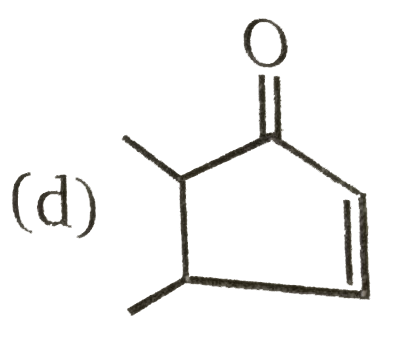
The correct IUPAC name of the compound:
CH3−CH3−CH3∣C=C−CH∣C2H6−CH3∣C−CH2−CH2−CH3A5-ethyl3,6-dimethyl non-3-eneB5-ethyl-4,7-dimethyl non-3-eneC4-methyl-5,7-diethyl oct-2-eneD2,4-ethyl-5-methyl oct-2-ene
The stability of given free radicals in decreasing order is
(i) CH3−.CH2 , (ii) CH3−C.H−CH3
(iii) CH3−.C∣CH3−CH3, (iv) .CH3
View SolutionThe IUPAC name of
CH3Ch2−H∣C∣CH3−C4H9∣C∣CH3−CH3
is
View SolutionThe stability of given free radicals in decreasing order is
(i) CH3−.CH2 , (ii) CH3−C.H−CH3
(iii) CH3−.C∣CH3−CH3, (iv) .CH3
View SolutionIUPAC name of CH3−H∣C∣C2H5−C4H9∣C∣CH3−CH3 is
View SolutionH2C=CH−CH3∣C∣CH3−COOHNaOH−−−−→CaO/ΔXwill be
View Solution