The pressure and volume of an ideal gas are related as pα1v2 for process A→B as shown in figure . The pressure and volume at A are 3p0andv0 respectively and pressure B is p0 the work done in the process A→B is found to be [x−√3]p0v0 find
![The pressure and volume of an ideal gas are related as p alpha 1/v^2 for process A rarrB as shown in figure . The pressure and volume at A are 3p0 and v0 respectively and pressure B is p0 the work done in the process ArarrB is found to be [x-sqrt(3)]p0v0 find](https://d10lpgp6xz60nq.cloudfront.net/physics_images/NTA_NEET_SET_41_E01_019_Q01.png)
3
6
4
5
The correct Answer is:A
Step by step video & image solution for The pressure and volume of an ideal gas are related as p alpha 1/v^2 for process A rarrB as shown in figure . The pressure and volume at A are 3p_0 and v_0 respectively and pressure B is p_0 the work done in the process ArarrB is found to be [x-sqrt(3)]p_0v_0 find by Physics experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
Similar Questions
Knowledge Check
The pressure p and volume V of an ideal gas both increase in a process.
Asuch a process is not possible.BThe work done by the system is positive.CThe tempreture of the system must increase.DHeat supplied to the gas is equal to the change in internal energy.The pressure P and volume V of an ideal gas both decreases in a process.
Asuch a process is not possible.BThe work done by the system is positive.CThe tempreture of the system must increase.DHeat supplied to the gas is equal to the change in internal energy.The internal energy (U), pressure (P) and volume (V) of an ideal gas are related as U=3PV+4. The gas is :-
ADiatomic onlyBPolyatomic onlyCEither monoatomic or diatomicDEither monoatomic or diatomic
The internal energy(U) ,pressure (P) and volume (V) of an ideal gas are related as U=3PV+4 .The gas is:
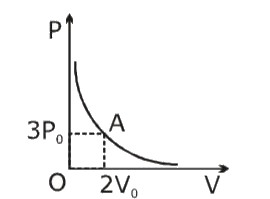
View SolutionThe variation of pressure P with volume V for an ideal monatomic gas during an adiabatic process is shown in figure . At point A the magnitude of rate of change of pressure with volume is
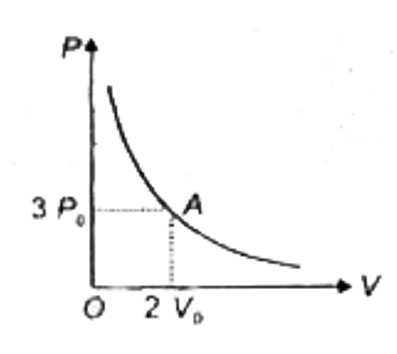
View SolutionThe variation of pressure P with volume V for an ideal monatomic gas during an adiabatic process is hown in figure. At the magnitude of rate of change of pressure with volume is-
View SolutionThe pressure and volume of a gas are P and V. If its pressure is reduced to P/2, by (A) isothermal process (B) by adiabatic process then the final volume will be :
View SolutionOne mole of an ideal monotomic gas undergoes a linear process from A to B in which its pressure p and its volume V change as shown in figure.
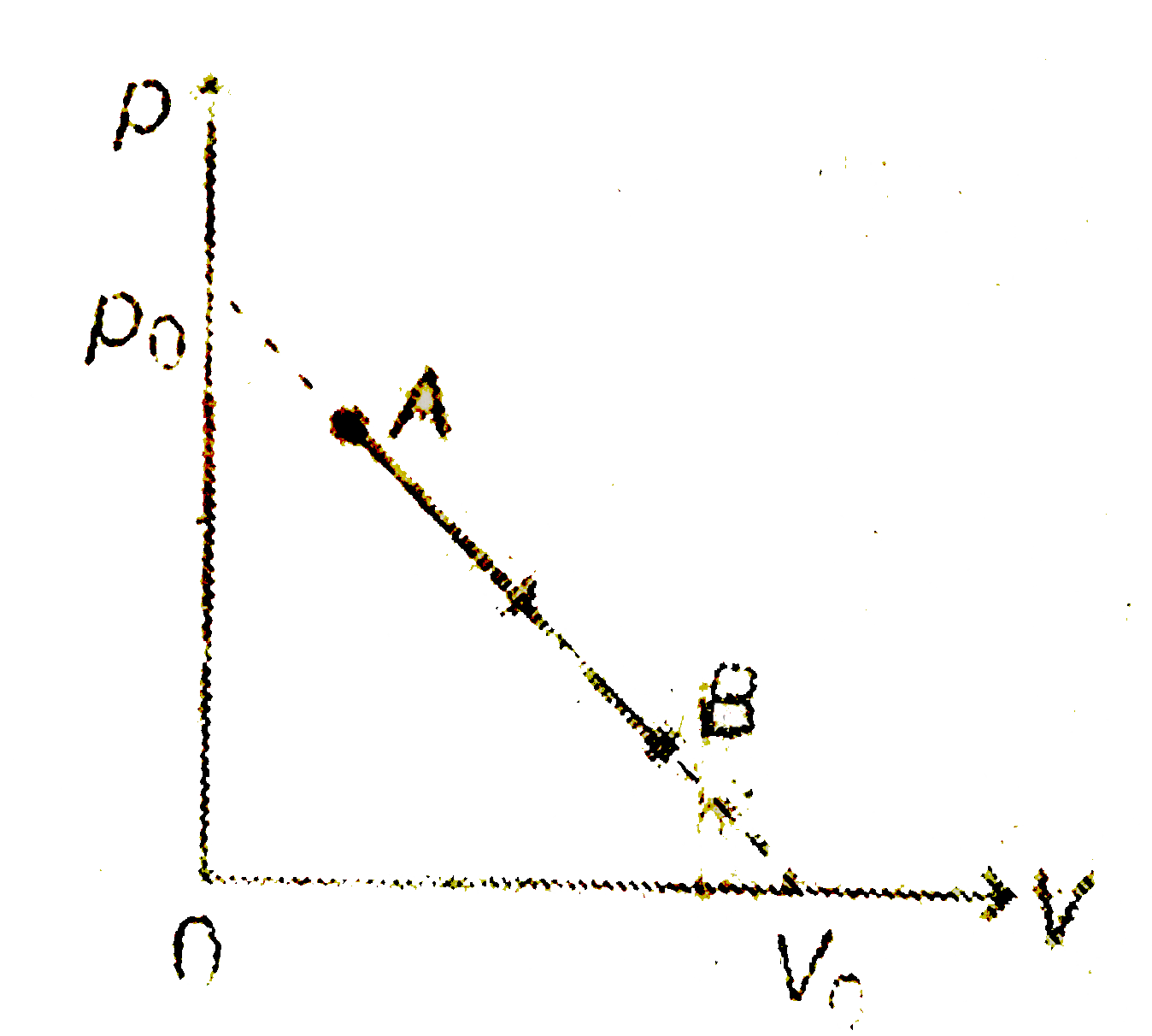
The absolute temperature T versus volume V for the given process is
View Solution

