In chemical adsorption, gas molecules are held to the surface by formation of chemical bond and nearly ........... is given out as heat of adsorption
400 kJ/mole
Topper's Solved these Questions
- SURFACE CHEMISTRYBOOK - FULL MARKSCHAPTER - SURFACE CHEMISTRYEXERCISE - ADDITIONAL QUESTIONS ( Match the column )
18 Videos
- SURFACE CHEMISTRYBOOK - FULL MARKSCHAPTER - SURFACE CHEMISTRYEXERCISE - ADDITIONAL QUESTIONS (Assertion and Reason )
15 Videos
- SURFACE CHEMISTRYBOOK - FULL MARKSCHAPTER - SURFACE CHEMISTRYEXERCISE - ADDITIONAL QUESTIONS ( Choose the correct answer and write it . )
150 Videos
- SOLID STATEBOOK - FULL MARKSCHAPTER - SOLID STATEEXERCISE - Additional Questions 5 Mark Questions
5 Videos
- TRANSITION AND INNER TRANSITION ELEMENTSBOOK - FULL MARKSCHAPTER - TRANSITION AND INNER TRANSITION ELEMENTSEXERCISE - Additional Questions (X. 5 Mark Questions.)
4 Videos
Similar Questions
Match the column I & II using the code
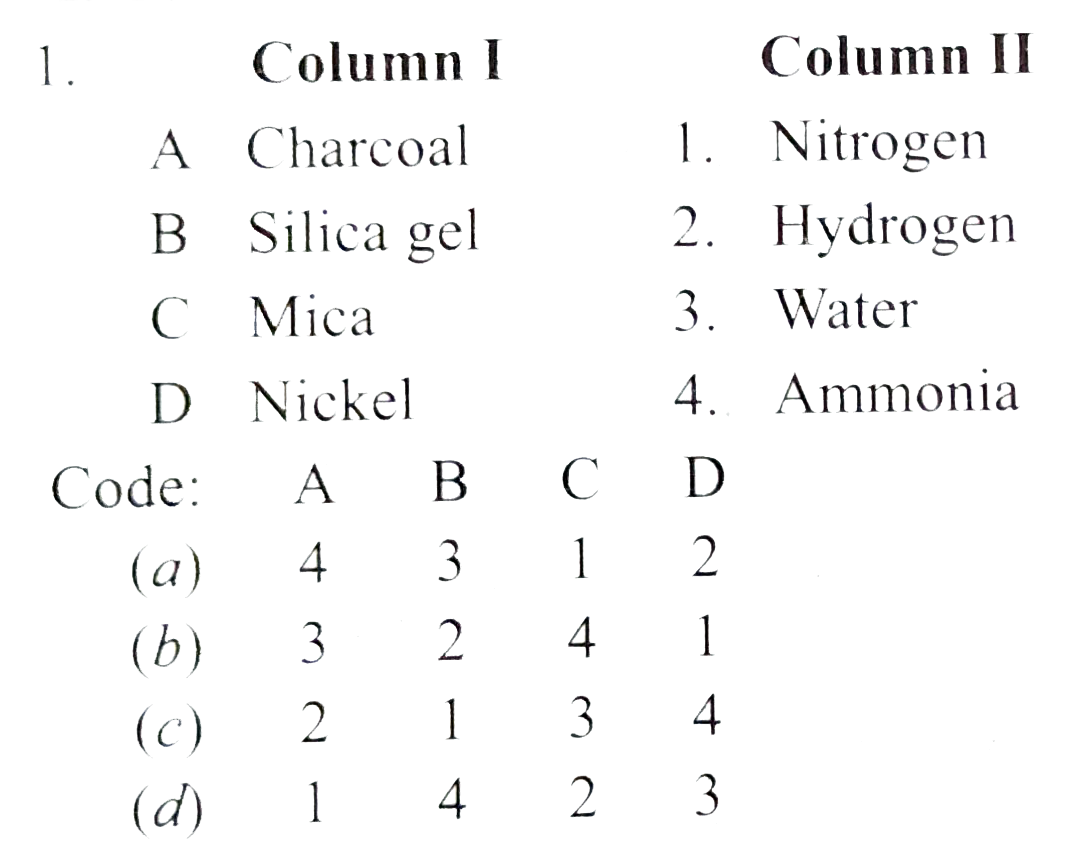
View SolutionAssertion (A) : Absorption is a bulk phenomenon.
Reason (R): The absorbed molecules are distributed throughout the absorbent.
View SolutionWhich one of the following is used to absorb colourants from sugar?
View SolutionWhat are general characteristics of solids ?
View SolutionExplain about the magnetic properties of transition elements,
View SolutionIn physical adsorption, the molecules of adsorbate are held by chemical forces.
View SolutionWhen gas molecules are held to the surface by the formation of chemical bond the heat energy released is nearly equal to ...............
View SolutionAssertion (A) : Chemical adsorption is an exothermic process. Reason (R) : In chemical adsorption, gas molecules are held to the surface by formation of chemical bonds. Since strong bond is formed, nearly 400kJ/mole is given out as heat adsorption.
View Solution(A)Enthalpy of physical adsorption is lower than chemical adsorption
(R ) Molecules of adsorbate and adsorbent are held together by vanderwall forces in physical adsorption and by chemical bonds in chemical adsorption
View SolutionRead the given passage and answers following questions :
There are mainly two type of adsorption of gases on solids. If accumulation of gases on the surface of solid occurs on account of weak vander waal forces, the adsorption is termed as physical adsorption. When gas molecules or atoms held to solid surface by chemical bonds, adsorption is termed as chemical adsorption. The chemical bonds may be covalent or ionic. Chemical adsorption involves a high energy of activation therefore it is referred as Activated adsorption. A physical adsorption at low temperature may pass into chemical adsorption temperature is increased.
What type of force exist in physical adsorption ?
View SolutionRead the given passage and answers following questions :
There are mainly two type of adsorption of gases on solids. If accumulation of gases on the surface of solid occurs on account of weak vander waal forces, the adsorption is termed as physical adsorption. When gas molecules or atoms held to solid surface by chemical bonds, adsorption is termed as chemical adsorption. The chemical bonds may be covalent or ionic. Chemical adsorption involves a high energy of activation therefore it is referred as Activated adsorption. A physical adsorption at low temperature may pass into chemical adsorption temperature is increased.
What are type of adsorption ?
View SolutionRead the given passage and answers following questions :
There are mainly two type of adsorption of gases on solids. If accumulation of gases on the surface of solid occurs on account of weak vander waal forces, the adsorption is termed as physical adsorption. When gas molecules or atoms held to solid surface by chemical bonds, adsorption is termed as chemical adsorption. The chemical bonds may be covalent or ionic. Chemical adsorption involves a high energy of activation therefore it is referred as Activated adsorption. A physical adsorption at low temperature may pass into chemical adsorption temperature is increased.
Give conditions of temperature which favours physical adsorption.
View SolutionRead the given passage and answers following questions :
There are mainly two type of adsorption of gases on solids. If accumulation of gases on the surface of solid occurs on account of weak vander waal forces, the adsorption is termed as physical adsorption. When gas molecules or atoms held to solid surface by chemical bonds, adsorption is termed as chemical adsorption. The chemical bonds may be covalent or ionic. Chemical adsorption involves a high energy of activation therefore it is referred as Activated adsorption. A physical adsorption at low temperature may pass into chemical adsorption temperature is increased.
What are type of adsorption ?
View SolutionRead the given passage and answers following questions :
There are mainly two type of adsorption of gases on solids. If accumulation of gases on the surface of solid occurs on account of weak vander waal forces, the adsorption is termed as physical adsorption. When gas molecules or atoms held to solid surface by chemical bonds, adsorption is termed as chemical adsorption. The chemical bonds may be covalent or ionic. Chemical adsorption involves a high energy of activation therefore it is referred as Activated adsorption. A physical adsorption at low temperature may pass into chemical adsorption temperature is increased.
What type of force exist in physical adsorption ?
View SolutionRead the given passage and answers following questions :
There are mainly two type of adsorption of gases on solids. If accumulation of gases on the surface of solid occurs on account of weak vander Waal forces, the adsorption is termed as physical adsorption. When gas molecules or atoms held to solid surface by chemical bonds, adsorption is termed as chemical adsorption. The chemical bonds may be covalent or ionic. Chemical adsorption involves a high energy of activation therefore it is referred as Activated adsorption. A physical adsorption at low temperature may pass into chemical adsorption temperature is increased.
How many types of adsorption of gases on solids?
View Solution

