1 mL of methyl acetate was added to 20 mL of 0.5 N HCl and 2 mL of the mixture was withdrawn from time to time during the process of hydorlysis of the ester and titrated with a solution of alkali . The amount of alkali needed for titration at various intervious intervals is given below :
Time020119∞(min)Alkali used19.2420.7326.642.03(mL)
Establish that the reaction is of first order .
aIntial concentrationCH3COOCH3+H2HCl−−−→[H1]CH3COOH+CH3+CH3OH(a−x)x concentration after time t
HCl acts as a catalyst .Since in every titration the amount of HCl is the same , the alkali used against HCl is subtracted from the total alkali used ( given in the data) to get the volume of alkali used only against CH3COOH . At zero time no CH3COOh is formed , so alkali used at zero time is only for HCl .
∴ we thus have ,
Time (min)020119∞Vol of alkali (mL)used:19.2420.7326.642.03againstCH3COOH−19.24=0−19.24−19.24=−19.24=1.497.3622.79(x)(x)(x)
Now following the same way as in Example 40 , we get the following results :
For t= 20 , k1=2.30320log.22.7922.79−1.49=0.0033−1min
For t = 119,k1=2.303119log.22.7922.79−7.36=0.0032−1min
The constancy of k1 shows that the reaction is of first order .
Topper's Solved these Questions
- CHEMICAL KINETICSBook:RC MUKHERJEEChapter:CHEMICAL KINETICSExercise:PROBLEMS
Explore 42 Videos
- CHEMICAL KINETICSBook:RC MUKHERJEEChapter:CHEMICAL KINETICSExercise:OBJECTIVE PROBLEMS
Explore 51 Videos
- CHEMICAL EQUIVALENCEBook:RC MUKHERJEEChapter:CHEMICAL EQUIVALENCEExercise:PROBLEMS
Explore 24 Videos
- CHEMICAL THERMODYNAMICSBook:RC MUKHERJEEChapter:CHEMICAL THERMODYNAMICSExercise:Objective problems
Explore 52 Videos
Similar Questions
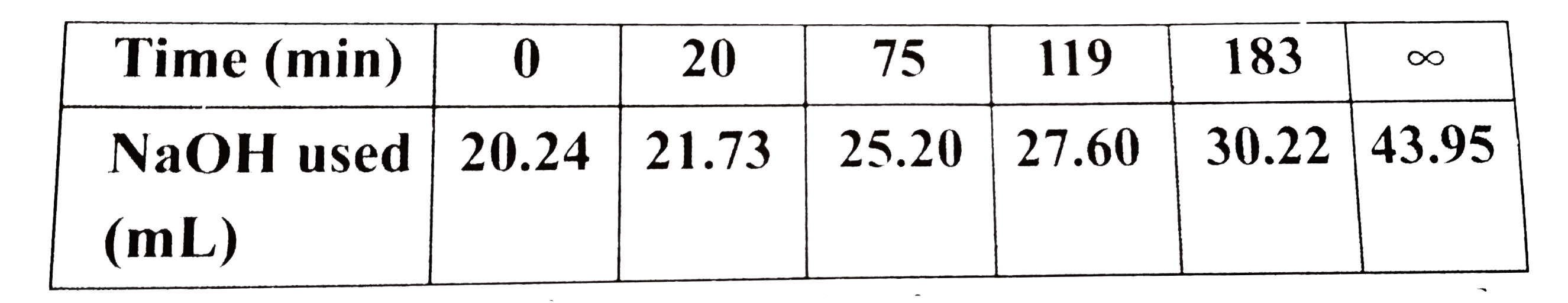
1.0 ml of ethyl acetate was added to 25 ml of N/2 HCl. 2 ml of the mixture were withdrawn from time to time during the progress of the hydrolysis of the ester and titrated against standard NaOH solution. The amount to NaOH required for titration at various intervals is given below :
Time (min):02075119183∞NaOH used (ml):20.2421.7325.2027.6030.2243.95
The value at ∞ time was obtained by completing the hydrolysis on boiling. Show that it is a reaction of the first order and find the average value of the velocity constant.
View Solution1.0mL of ethyl acetate was added to 25mL of N/2HCl,2mL of the mixture were withdraw form time to time during the progress of the hydrolyiss of the ester and titrated against standard NaOH solution. The amount of NaOH required for titration at various intervals is given below:
The value at ∞ time was obtained by completing the hydrolyiss on boiling. Show that it is a reaction of the first order and find the average value of the velocity constant.
View Solution
Knowledge Check
20mL of 0.5 M HCl and 35 mL of 0.1 N NaOH are mixed. The resulting solution will
ABe neutralBBe basicCTurn phenolphthalein solution pinkDTurn methyl orange red20mL of 0.5 M HCl and 35 mL of 0.1 N NaOH are mixed. The resulting solution will
Abe neutralBbe basicCturn phenolphthalein solution pinkDturn methyl orange red20mL of 0.5 M HCl and 35 mL of 0.1 N NaOH are mixed. The resulting solution will
Abe neutralBbe basicCturn phenolphthaleinDturn methyl orange solution red
1.0mL of ethyl acetate was added to 25mL of N/2HCl,2mL of the mixture were withdraw form time to time during the progress of the hydrolyiss of the ester and titrated against standard NaOH solution. The amount of NaOH required for titration at various intervals is given below:

The value at ∞ time was obtained by completing the hydrolyiss on boiling. Show that it is a reaction of the first order and find the average value of the velocity constant.
View Solution1.0 mL of ethyl was added to 25 mL of N2 HCl, 2.0mL of the mixture was withdrawn from time to time during the progress of easter hydrolysis and titrated against standard NaOH solutions. The amount of NaOH required for titration at various intervals is given below:

The value of at infinite time was obtained by completing the hydrolysis on boiling. Show that the reaction is of first order. Also find the average value of rate constant.
View SolutionThe following data were obtained for the saponification of ethyl acetate using equal concentration of ester and alkali .
Time041020(min)Vol of acid used8.045.303.502.22(mL)
Show that the reaction is of second order .
View Solution1 mL of methyl acetate was added to a flash containing 20 mL of N/20 HCl maintained at 25∘C. 2mL of the reaction mixture were withdrawn at different intervals and titrate with a standard alkali solution. The following results were obtained :
Time (min)075119183∞Alkali used (mL)19.2424.2026.6029.3242.03
show that the reaction follows first order kinetics
View SolutionMethyl acetate was subjected to hydrolyiss in N−HCl at 298K. 5mL of the mixture is withdraw at different intervals and titrated with constant with about N/8NaOH. The following results were obtained:
∣∣∣Time (min)0254061∞Vol of alkali used (mL)19.2424.2026.6029.3242.03∣∣∣
Show that the reaction is of first order.
View Solution

