Assertion (A) Separation of Zr and Hf is difficult.
Reason (R ) Because Zr and Hf lie in the same group of the Periodic Table.
Both assertion and reason are true and reason is the correct explanation of the assertion .
Both assertion and reason are true but reason is not the correc explanation of assertion.
Assertion is not true for reason is true
Both assertion and reason are false.
The correct Answer is:b
Correct explanation . Zr and Hf have nearly the same size.
Topper's Solved these Questions
- D- AND F-BLOCK ELEMENTSBook:PRADEEPChapter:D- AND F-BLOCK ELEMENTSExercise:LONG ANSWER QUESTIONS
Explore 8 Videos
- D- AND F-BLOCK ELEMENTSBook:PRADEEPChapter:D- AND F-BLOCK ELEMENTSExercise:ADDITIONAL QUESTIONS (VERY SHORT ANSWER QUESTIONS)
Explore 49 Videos
- D- AND F-BLOCK ELEMENTSBook:PRADEEPChapter:D- AND F-BLOCK ELEMENTSExercise:MATCHING TYPE QUESTIONS
Explore 9 Videos
- CORDINATION COMPOUNDSBook:PRADEEPChapter:CORDINATION COMPOUNDSExercise:Important Questions
Explore 28 Videos
- ELECTROCHEMISTRYBook:PRADEEPChapter:ELECTROCHEMISTRYExercise:IMPORTANT QUESTIONS FOR BOARD EXAMINATION
Explore 25 Videos
Similar Questions
Identify A to E and also explain the reactions involved.
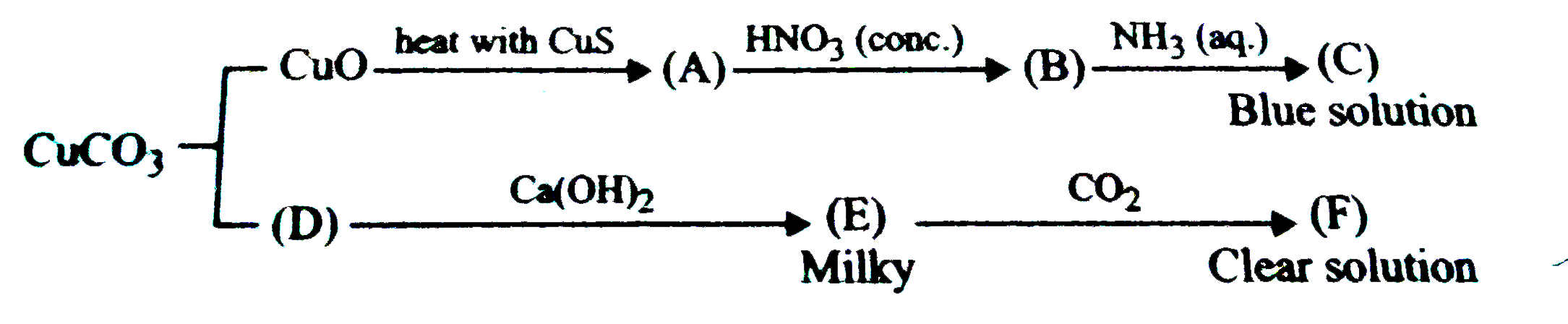
View SolutionThe transition elements are so named because
View SolutionMatch the catalyst given in Column I with the processes given in Column II.

View SolutionWhat aer ambidentate ligands? Give an example.
View SolutionA current is passed through two cells connected in series. The first cell contains X(NO3)3(aq) and the second cell contains Y(NO3)2(aq) . The relative atomic masses of X and Y are in the ratio 1:2. What is the ratio of liberated mass of X to that of Y ?
View SolutionAssertion (A) Separation of Zr and Hf is difficult. Reason (R ) Because Zr and Hf lie in the same group of the Periodic Table.
View SolutionAssertion. Separation of Zr and Hf is difficult. Reason.Zr and Hf lie in the same group of the periodic table.
View SolutionThe properties of Zr and Hf are same because
View SolutionAssertion : Zr and Hf occur together in nature andare difficult to separate.
Reason : Zr and Hf have identical radii due to lanthanide contraction.
View SolutionAssertion (A) : Separation of Zr and Hf is difficult. Reason (R ) : Zr and Hf lies in the same group of the periodic table.
View SolutionAssertion: Separation of Zr and Hf is difficult. Reason : Because Zr and Hf lie in the same graph of the periodic table.
View Solutionअभिकथन: Zr एवं Hf प्रकृति में साथ-साथ पाये जाते हैं तथा उन्हें पृथक करना कठिन होता है
तर्क: Zr एवं Hf में लैन्थेनॉइड संकुचन के कारण समरूप त्रिज्याएँ होती हैं।
View Solution(A): Zr and Hf have nearly equal atomic radii.
(R): Zr and Hf belong to the same group.
View SolutionAssertion (A): Separation of Zr and Hf is difficult.
Reason (R ): Because Zr and Hf lie in the same group of the periodic table.
View SolutionThe properties of Zr and Hf are similar because :
View Solution
