2RCl+SiCu power−−−−−→570 KR2SiCl2H2O−−→R2Si(OH2)Polymerisation−−−−−−−−→X, Then X will be
Linear long chain silicone
Cyclic silicone
Cross linked silicone
none of these
The correct Answer is:A
To solve the given question, we will follow the chemical reactions step by step and analyze the products formed at each stage.
Step 1: Reaction of RCl with Si
The first reaction involves the combination of 2 moles of RCl with silicon (Si) in the presence of copper powder at 570 K. This reaction can be represented as:
2RCl+SiCu, 570 K−−−−−−→R2SiCl2
Here, RCl represents an organic halide, and R2SiCl2 is a silicon compound where silicon is bonded to two R groups and two chlorine atoms.
Hint for Step 1: Identify the reactants and products based on the reaction conditions provided.
Step 2: Hydrolysis of R2SiCl2
Next, the product R2SiCl2 undergoes hydrolysis in the presence of water (H2O):
R2SiCl2+2H2O→R2Si(OH)2+2HCl
In this step, the chlorine atoms are replaced by hydroxyl (OH) groups, leading to the formation of R2Si(OH)2, which is a silanol compound.
Hint for Step 2: Understand the process of hydrolysis and how it replaces halogens with hydroxyl groups.
Step 3: Polymerization of R2Si(OH)2
The final step involves the polymerization of the silanol compound R2Si(OH)2. During polymerization, the hydroxyl groups can react with each other, leading to the formation of siloxane bonds (Si-O-Si) and the elimination of water molecules:
nR2Si(OH)2→(R2Si−O)n+nH2O
This process results in the formation of a polymeric structure, which is typically linear or branched.
Hint for Step 3: Recognize that polymerization can lead to different structures, but in this case, it primarily produces linear or branched chains.
Conclusion
Based on the above steps, the final product X from the polymerization of R2Si(OH)2 will be a linear chain silicone compound. Therefore, the correct answer is:
X will be a linear chain silicone.
Final Answer
X = Linear chain silicone
The first reaction involves the combination of 2 moles of RCl with silicon (Si) in the presence of copper powder at 570 K. This reaction can be represented as:
Next, the product R2SiCl2 undergoes hydrolysis in the presence of water (H2O):
The final step involves the polymerization of the silanol compound R2Si(OH)2. During polymerization, the hydroxyl groups can react with each other, leading to the formation of siloxane bonds (Si-O-Si) and the elimination of water molecules:
Based on the above steps, the final product X from the polymerization of R2Si(OH)2 will be a linear chain silicone compound. Therefore, the correct answer is:
X = Linear chain silicone
Similar Questions
Complete the following:
a.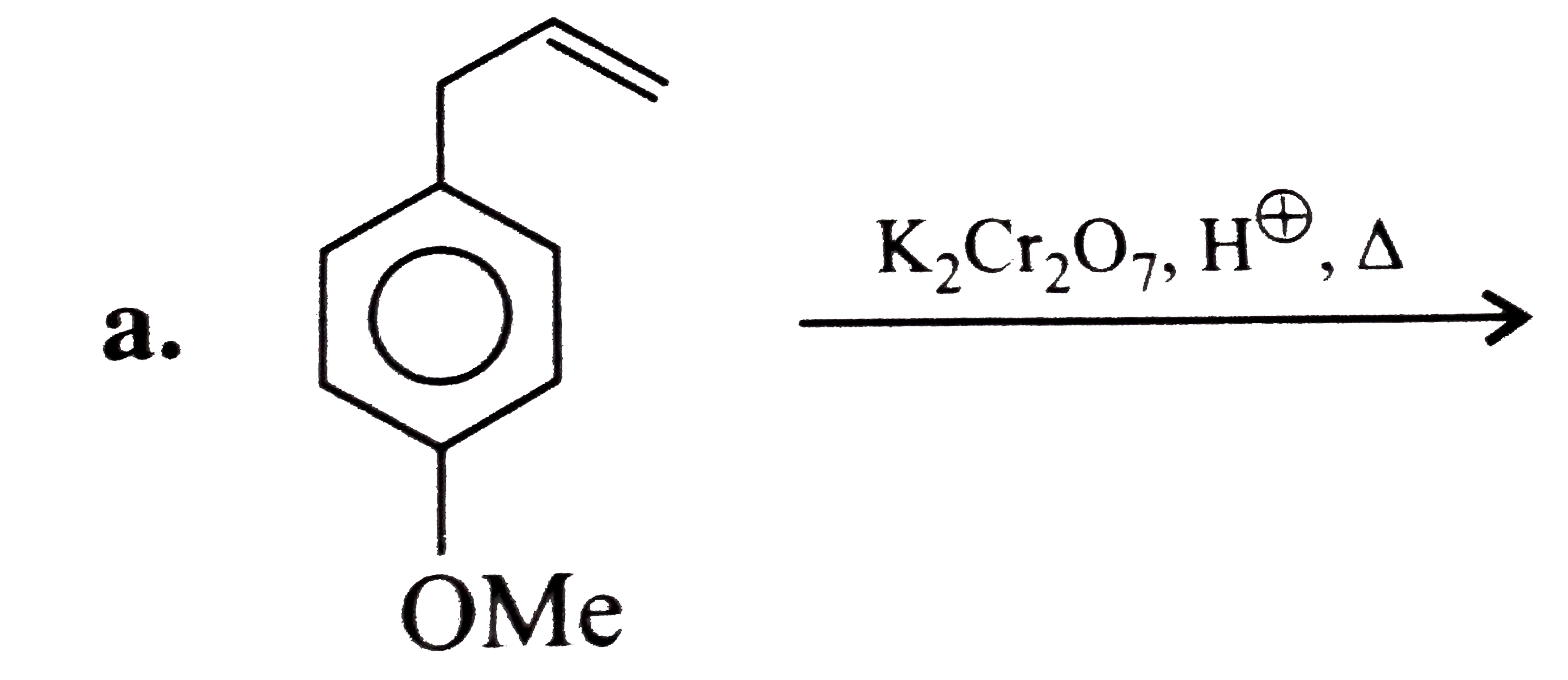
b.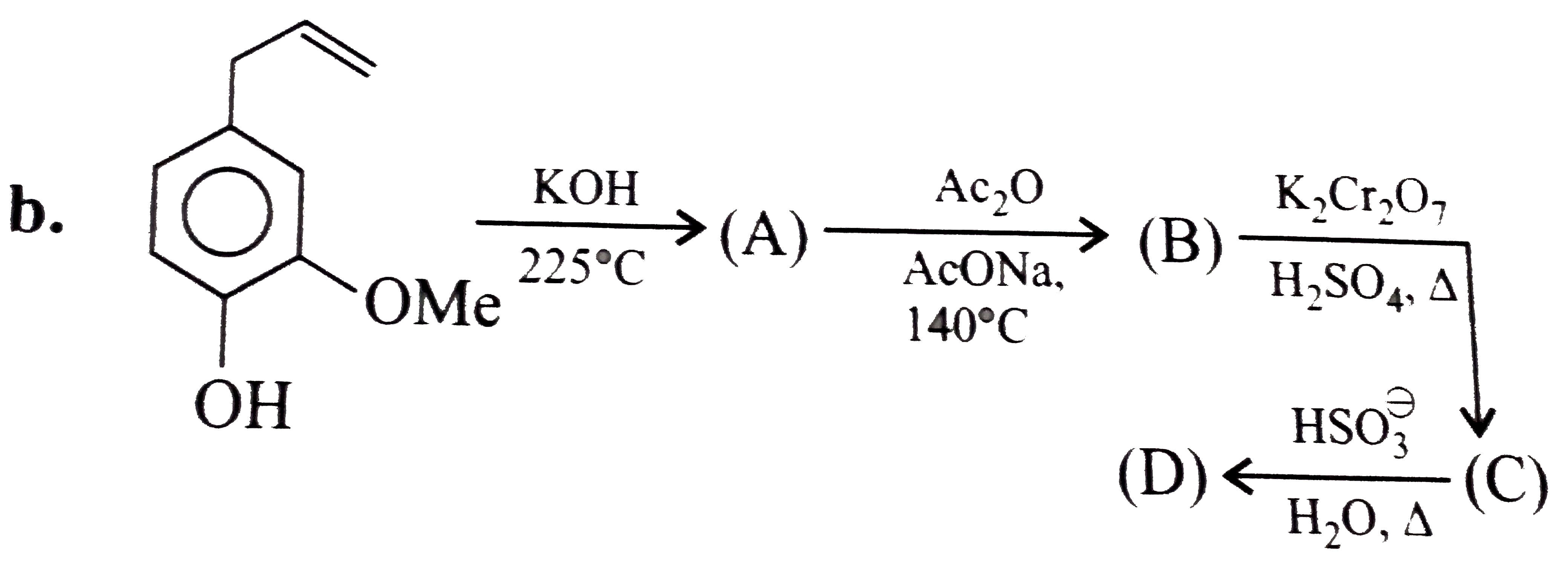
c.
d.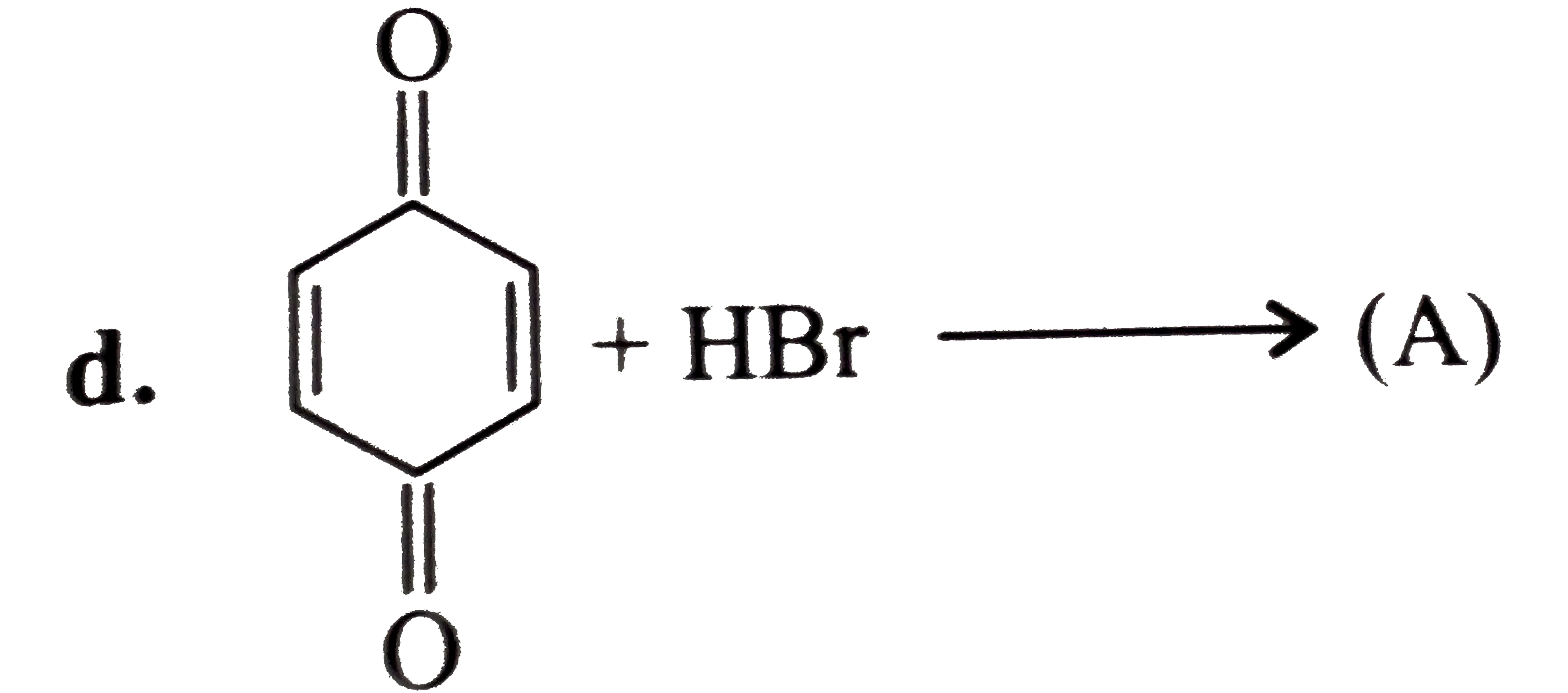
e.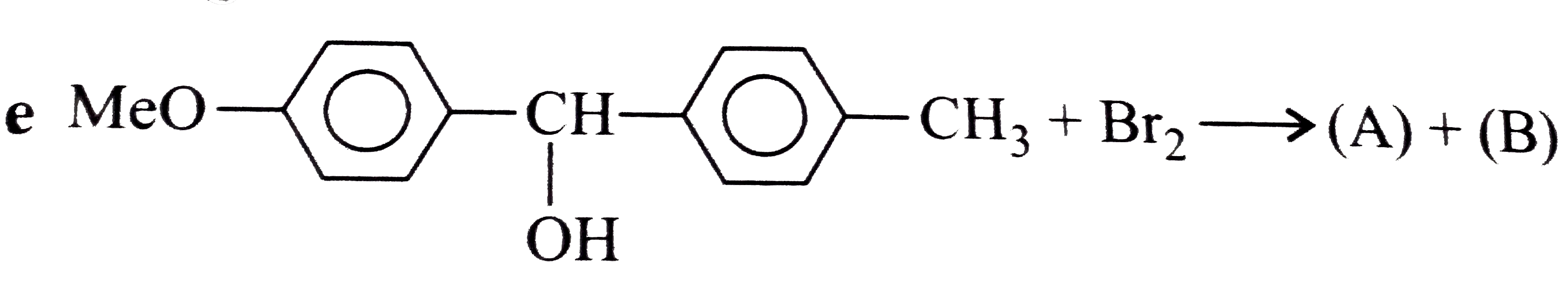
f.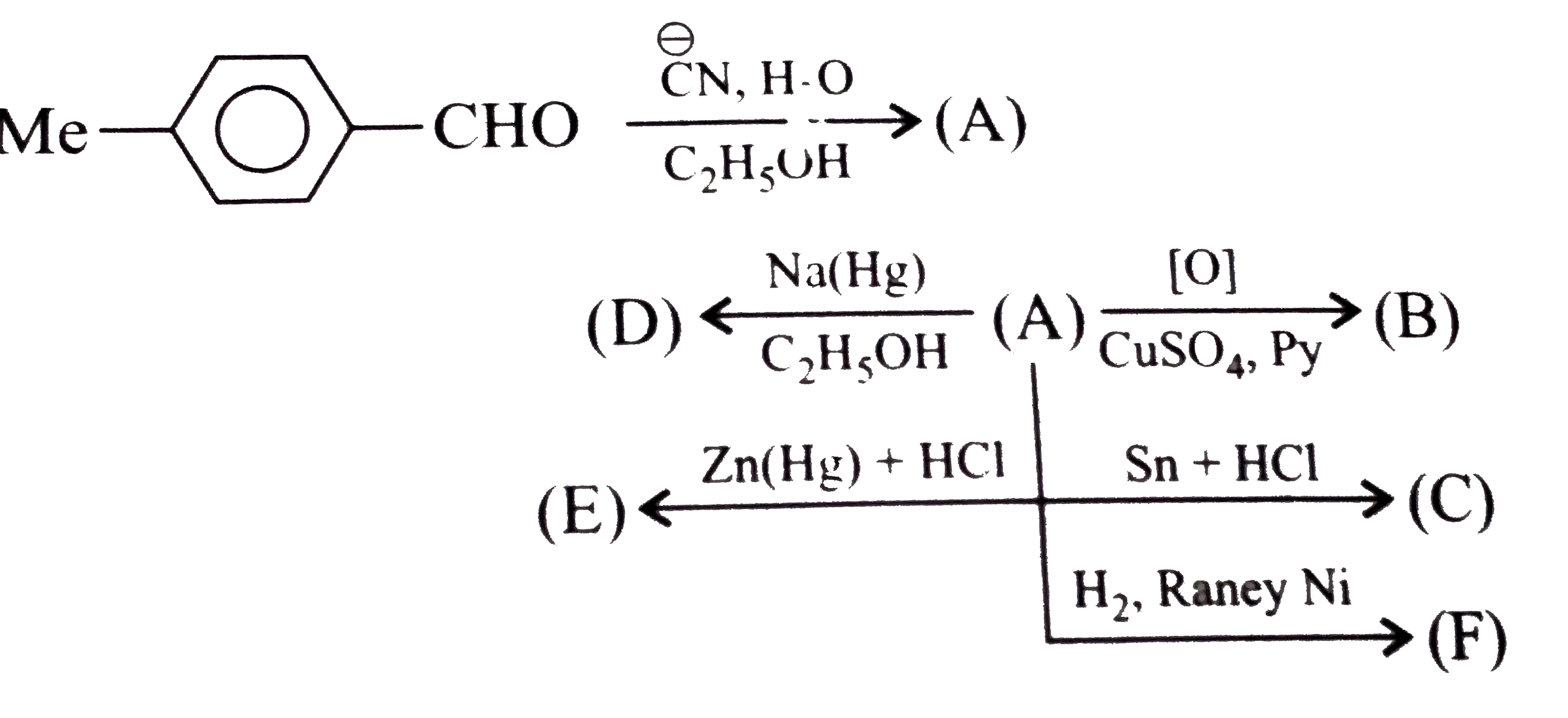
g.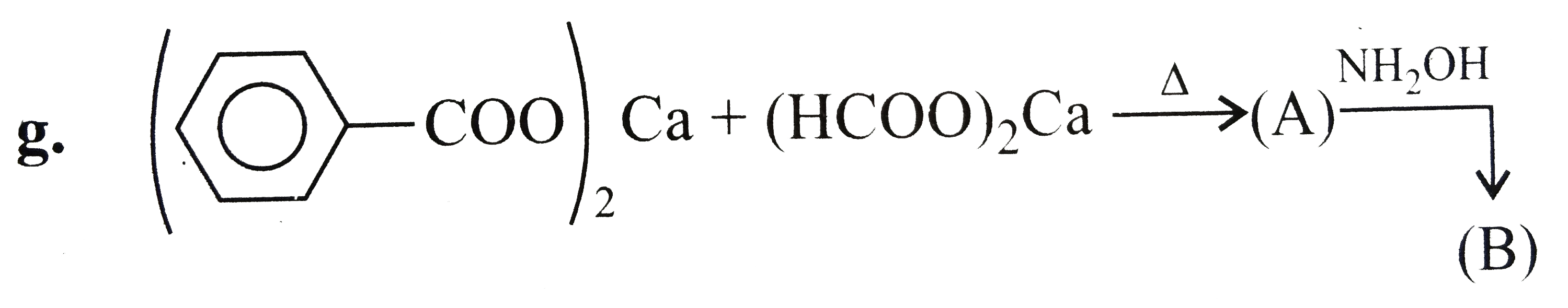
h. PhCHO+Cl2No catalyst−−−−−−→(A)
i.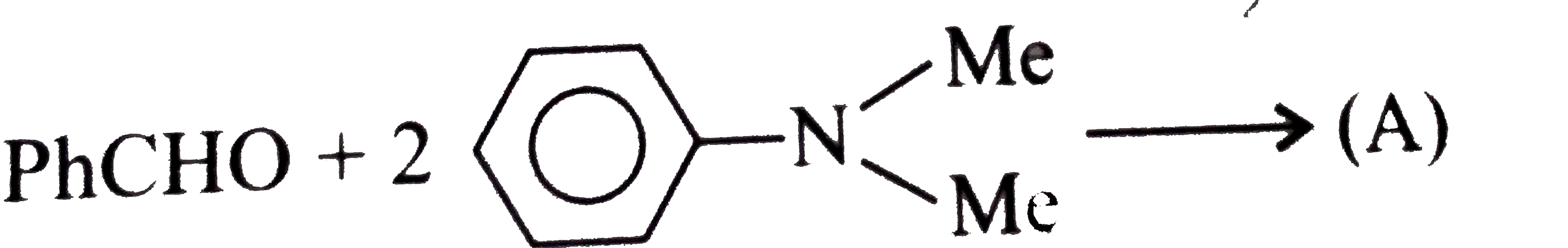
j. PhCHO+(C2H5CO)2OC2H5COONa−−−−−−−→A
k.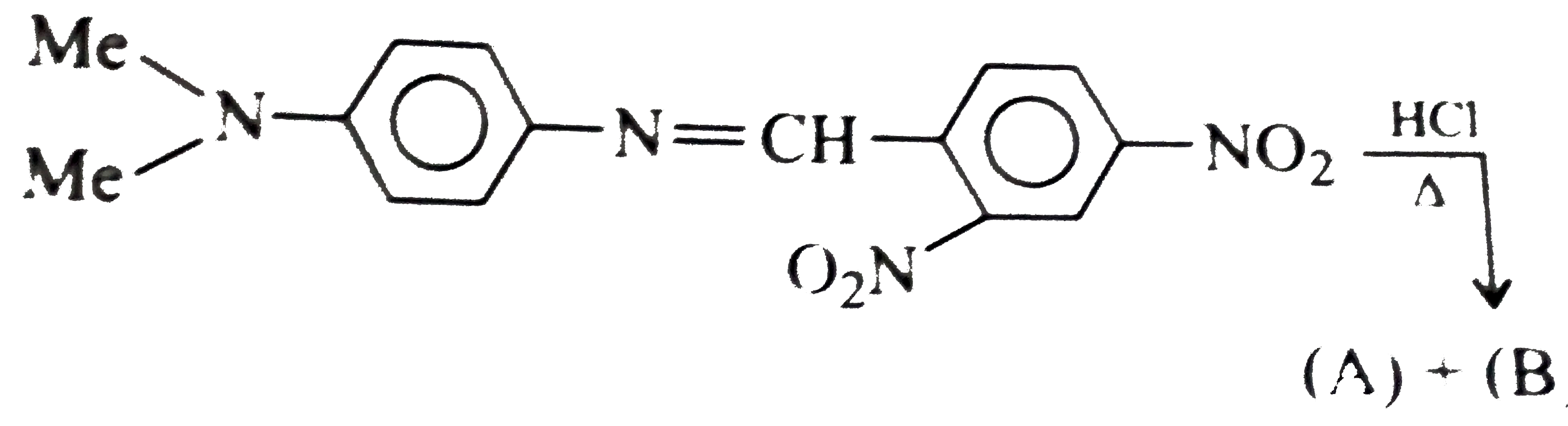
l.
m.
n.
o.
p.
q.
r.
s. PhCOCH3+SeO2→(A)
t. PhCH=CH−O∣∣C−Ph+KCNCH3COOH−−−−−−→(A)
u. Ph−C∣CNH−O∣∣C−CH3(i)H3O⊕−−−−−→(ii)Δ(A)
v.
w. Ph−OH∣C∣Ph−CH2NH2HNO2−−−→(A)Oxidation−−−−−→(B)
x. PhCOPh+KOH→(A)+(B)
y.
z.
View SolutionRCIcu−powder−−−−−−→SiR2SiCL2H2O−−→R2Si(OH)2condensation−−−−−−−→A
Compound (A) is
View Solution
Knowledge Check
2RCl + Si overset("Cu पूर्ण")underset(570 K) to R_(2)SiCl_(2) overset(H_(2)O)to R_(2)Si(OH)_(2) overset("बहुलीकरण")to X, तब होगा-
Aरैखिक दीर्घ श्रृंखला सिलिकॉनBचक्रीय सिलिकॉनCतिर्यक बंधित सिलिकॉनDइनमे से कोई नहीं2RMgCl+SiCl4H2O−−→Bpolymerisation−−−−−−−−→C.C is
Acyclic siliconeBlinear siliconeCcross linked siliconeDSiO22RCl + Si overset(Cu "चूर्ण")underset(570 K) to R_(2)SiCl_(2) overset(H_(2)O) to R_(2)Si(OH)_(2) overset("बहुलकीकरण") to (A)
निम्न अभिक्रिया में A क्या होगा?Aचक्रीय सिलिकॉनBतिर्यक बंधित सिलिकॉनCरैखिक सिलिकॉनDइनमें से कोई नहीं
CH3Cl+SiCuPowder−−−−−−→570X2H2O−−→Y
Y & X respectively are
View Solution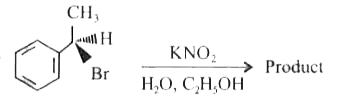 KNO2−−−−−−−−→H2O,C2H5OH Product
KNO2−−−−−−−−→H2O,C2H5OH Product
The main product is
View SolutionIn the following reaction, XBromination−−−−−−→YNaNO2−−−−→HClZBoiling−−−−→C2H5OH Tribomobenzene, X is
View SolutionPhenol (i) NaOH−−−−→(ii) CO2(A)H+/H2O−−−−−→(B)Ac2O−−→(C)
In this reaction , identify the incorrect statement?
View SolutionC2H5IKOH(alc)−−−−−−→ACl2(g)−−−→770KX, X can be
View Solution

