A flux is often added to remove impurities from a concentrated ore. In the reaction
CaO+SiO2→CaSiO3
the slag and the flux are
SiO2andCaSiO3
CaOandSiO2
CaSiSO2andCaO
both (1) and (3)
The correct Answer is:a
The ores even after concentration , contain some ganghue.To remove these gangue particle cortain substance are mixed to form a fushible ore which combite with the gangue to form a fusible meterial which is not solution in molten metal . The sulstances used are called fluxed and the fasible merterial formed during reduction process is called slag
Slag is usually lighly and thus on the surface of the moltes metal
SiO2acidic flux+CaObasicimpurity(gangue)→CaSiO3slag
Flaxtes are classified as (i) acidic flox and (ii) basic , flex An acoidic flux siles bores etc is the chenical substans which removes the imparities.
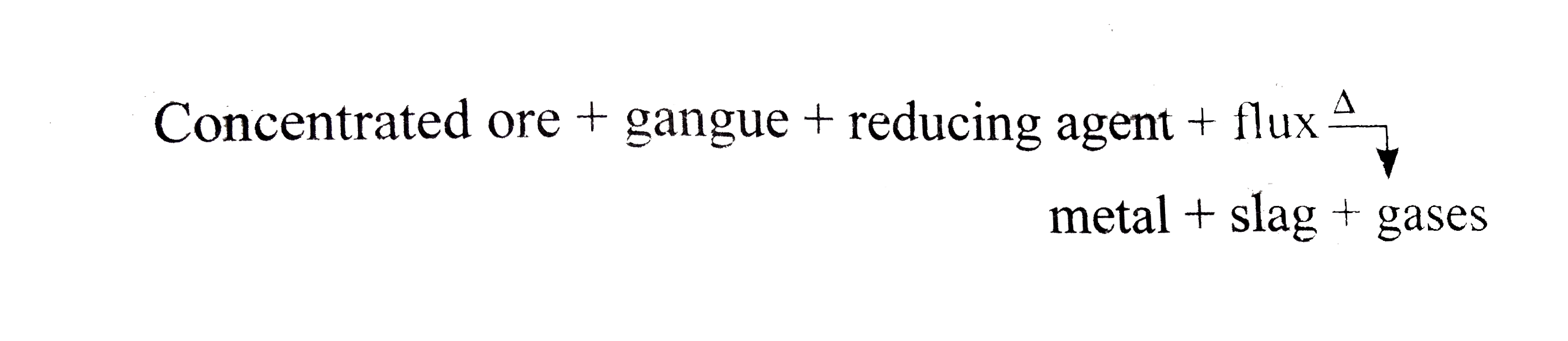
The basic flax (e.g. line limestrons ,magnetics ferric oxide, atc) is the chemecal substance which removes the acide imurites
Similar Questions
How many reaction can show slag formation process from the given reaction ?
(i) SiO2+CaO→CaSiO3
(ii) FeO+SiO2→FeSiO3
(iii) CaO+P2O5→Ca3(PO4)2
(iv) Cr2O3+2Al→Al2O3+2Cr
(v) MgCO3+SiO2→MgSiO3+CO2.
View SolutionBalance the following chemical equations and identify the type of chemical reaction.
CaO(s)+SiO2(s)→CaSiO3(s)
View Solution
Knowledge Check
For the reaction CaCO3(s)→CaO(S)+CO2(g) the entropy
AincreasesBdecreasesCremains unchangedDchange cannot be predictedName the flux to remove the impurity of SiO2
AP4O10BCaOCN2O5DAI2O3The equilibrium constant for the given reaction,
CaCO3(g)→CaOs+CO2(g) is given by :AKc=[CaO]⋅[CO2][CaCo3]BKc=[CaO][CaCO3]CKc=[CO2]DKc=[CaO][CO2]
Identity the endothermic and exothermic reaction.
CaCO3(s)Δ−−→CaO(s)+CO2↑⏐⏐
View SolutionIf an ore contains impurity of SiO2, identify the appropriate flux for the removal of it
View SolutionFor the reaction, CaCO3(s)→CaO(s)+CO2(g), which is the correct representation?
View SolutionIn coal, pyrites (FeS2) is present as a pollution-causing impurity, which is removed by combustion.
2FeS2+5O2→4SO2+2FeO.
A process designed to remove orgainc sulphur from coal prior to combustion involves the reaction.
X−S−Y+2NaOH→X−O−Y+Na2S+H2O
CaCO3→CaO+CO2
Na2S+CO2+H2O→Na2CO3+H2S
CaO+H2O→Ca(OH)2
Na2CO3+Ca(OH)2→CaCO3+2NaOH
In the processing in 320 metric tons of a coal having 1.0% sulphur content, how much limestone (CaCO3) must be edecomposed to provied enough Ca(OH)2 to regenerate the NaOH used in the original leaching step?
View SolutionExtraction of Al from bauxite is carried out by various stages in Hall's process which involves
I removal of sand and heavier impurities by gravity separation method.
II. removal of magnetic impurities by magnetic separator.
III. fusing the concentrated finely divided ore with Na2CO3 and CaCO3 and then extracting with H2O.
IV. ignition at 1100∘C.
V. passing CO2
Correct order of these steps are
View Solution
