A teritary alcohol H upon acid catalysed dehydration gives a product I. Ozonolysis of 1 leads to compounds J and K. Compound J upon reaction wth KOH gives benzyl alcohol and compound L, whereas K on reaction with KOH gives only M, having following structure
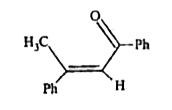
The structure of compound J, K and L respectively, are
PhCOCH3,PhCH2COCH2andPhCH2COO−K+
PhCOCH3,PhCH2CHOandPhCOO−K+
PhCOCH3,PhCH2CHOandCH3COO−K+
PhCOCH3,PhCOCH2andPhCOO−K+
The correct Answer is:D
The structure of compound J, K and L respectively are
Ph−CHO(J),Ph−C∣CH3=O(K)andPhCOO−K+(L)
Topper's Solved these Questions
- ALCOHOLS, PHENOLS AND ETHERSBOOK - BRILLIANT PUBLICATIONCHAPTER - ALCOHOLS, PHENOLS AND ETHERSEXERCISE - LEVEL III (Statement Type Questions)
8 Videos
- ALCOHOL, PHENOL & ETHER BOOK - BRILLIANT PUBLICATIONCHAPTER - ALCOHOL, PHENOL & ETHER EXERCISE - LEVEL II (ASSERTION - REASON TYPE)
40 Videos
- ALDEHYDES AND KETONES BOOK - BRILLIANT PUBLICATIONCHAPTER - ALDEHYDES AND KETONES EXERCISE - LEVEL-II
42 Videos
Similar Questions
Statement 1 : The water solubility of the alcohols follow the order, ter-butyl alcohol > sec-butyl alcohol > n-butyl alcohol.
Statement 2 : Alcohols form H-bonding with water to show soluble nature.
View SolutionAssertion : Carbon oxygen bond length of phenol is slightly less than that in methanol.
Reason : There exist a partial double bond character and carbon to which oxygen is attached in phenol is sp2 hybridised.
View SolutionThe general formula of aliphatic saturated aldehydes and ketones is:
View SolutionA tertiary alcohol (H) upon acid-catalysed dehydration gives a product (I). Ozonolysis of (I) leads to compounds (J) and (K). Compound (J) upon reaction with KOH gives benzyl alcohol and a compound (L), whereas (K) on reaction with KOH gives only (M). Compound (H) is formed by the reaction of:
View SolutionA tertiary alcohol (H) upon acid-catalysed dehydration gives a product (I). Ozonolysis of (I) leads to compounds (J) and (K). Compound (J) upon reaction with KOH gives benzyl alcohol and a compound (L), whereas (K) on reaction with KOH gives only (M). The structurer of compound (I) is:
View SolutionA tertiary alcohol (H) upon acid-catalysed dehydration gives a product (I). Ozonolysis of (I) leads to compounds (J) and (K). Compound (J) upon reaction with KOH gives benzyl alcohol and a compound (L), whereas (K) on reaction with KOH gives only (M). The structures of compounds (J), (K), and (L), respectively, are:
View SolutionA tertiary alcohol [H] upon acid catalysed dehydration gives a product [I]. Ozonolysis of [I] leads to compounds [J] to [K]. The compound [J] upon reactions with KOH gives benzyl alcohol a compound [L] wheras [K} on reaction with KOH gives only [M]. The compund [M] has the formula.
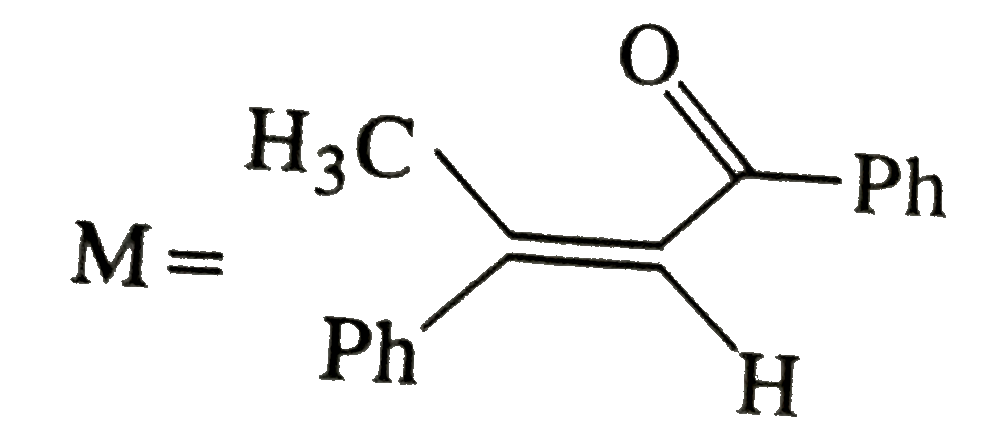
The structure of compound [I] is
View SolutionA tertiary alcohol [H] upon acid catalysed dehydration gives a product [I]. Ozonolysis of [I] leads to compounds [J] to [K]. The compound [J] upon reactions with KOH gives benzyl alcohol a compound [L] wheras [K} on reaction with KOH gives only [M]. The compund [M] has the formula.
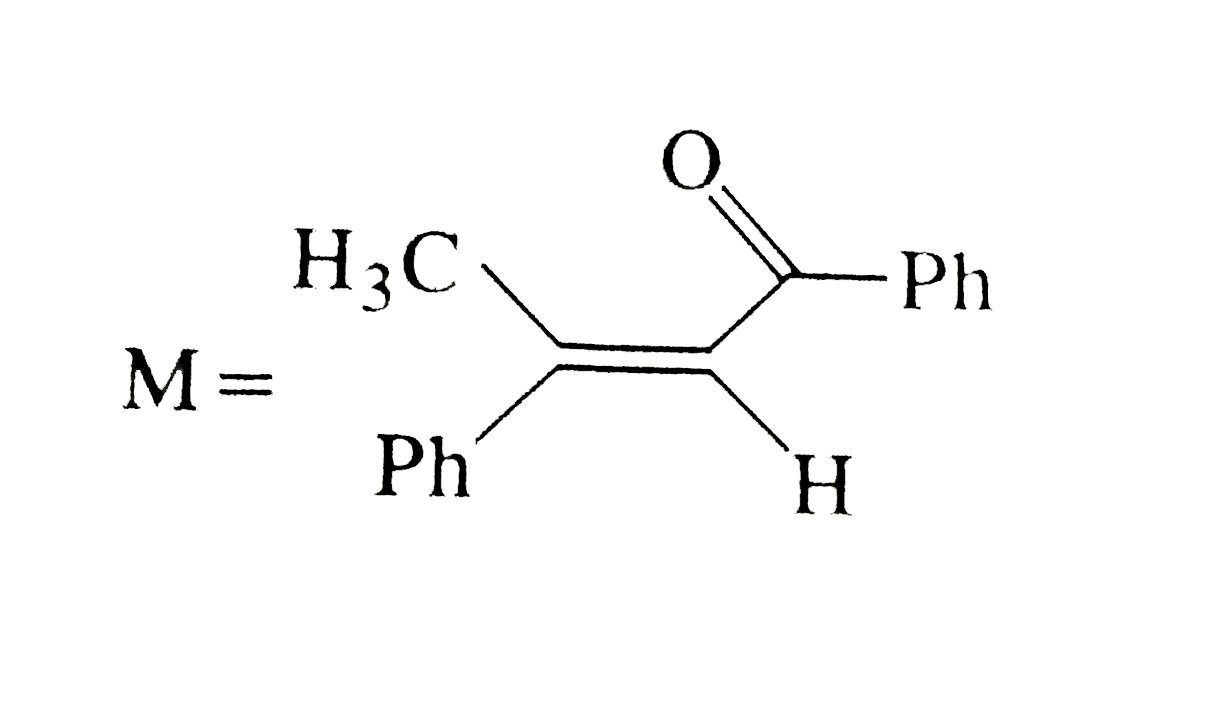
The structure of compounds [J],[K] and [L] are respectively :
View SolutionA tertiary alcohol (H) upon acid-catalysed dehydration gives a product (I). Ozonolysis of (I) leads to compounds (J) and (K). Compound (J) upon reaction with KOH gives benzyl alcohol and a compound (L), whereas (K) on reaction with KOH gives only (M).
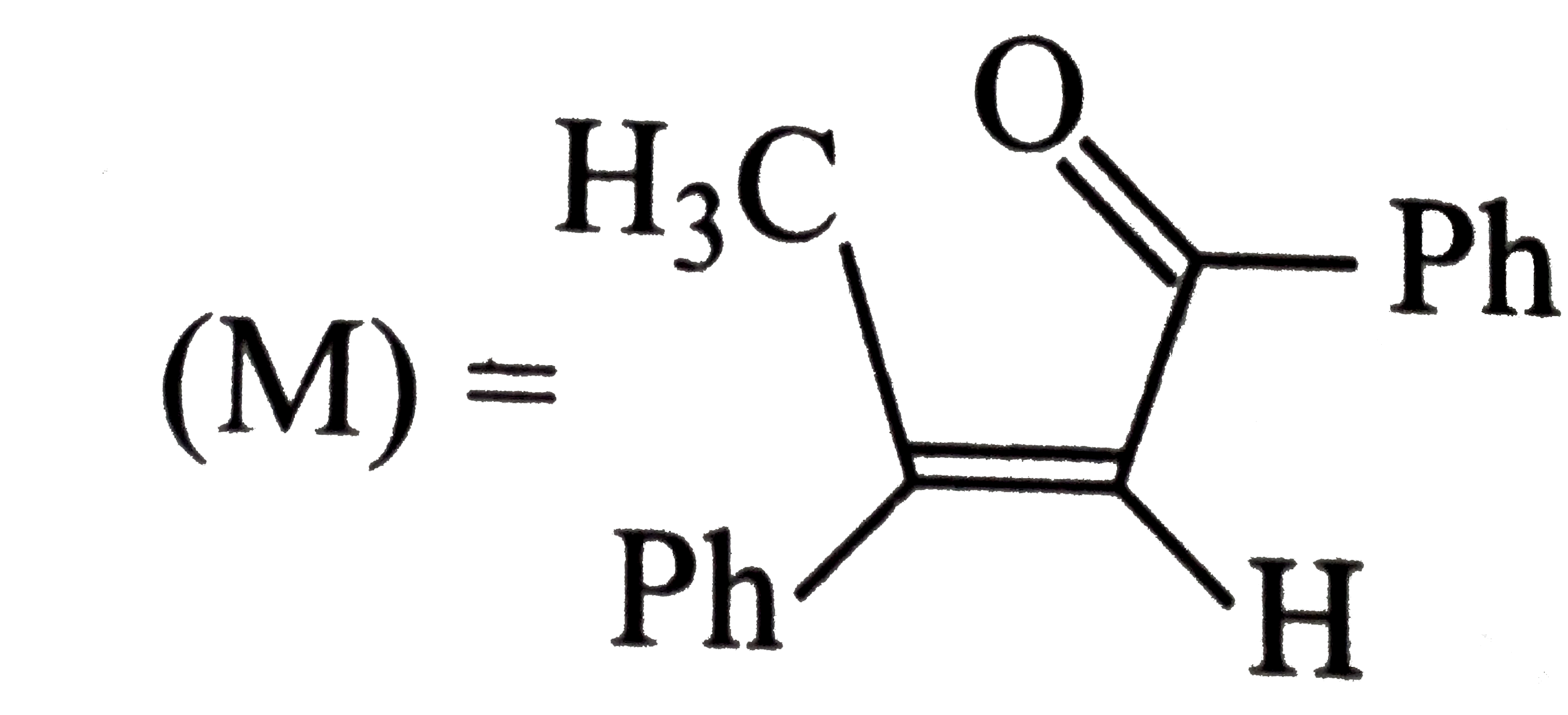
The structurer of compound (I) is:
View SolutionA tertiary alcohol H upon acid- catalyzed dehydration gives a product I. Ozonolysis of to compounds J and K. Compound J upon reaction with KOH gives benzly alcohol and a compound L, whereas K on reaction with KOH gives only M.

The structure of compound I is
View SolutionA tertiary alcohol H upon acid- catalyzed dehydration gives a product I. Ozonolysis of to compounds J and K. Compound J upon reaction with KOH gives benzly alcohol and a compound L, whereas K on reaction with KOH gives only M. The structures of compound J,K and L respectively , are
View SolutionA tertiary alcohol H on acid catalysed dehydration gives a product I. Ozonolysis of I leads to compounds. J and K . Compound J upon reaction. With KOH gives benzyl alcohol and a compound L, whereas K on reaction with KOH gives only. M The structure of compound I is :
View Solutionएक तृतीयक ऐल्कोहॉल H अम्ल उत्प्रेरित निर्जलीकरण पर उत्पाद I देता है | I ओजोनी - अपघटन पर यौगिक J तथा K देता है | यौगिक J,KOH के साथ अभिक्रिया करने पर बेंजील ऐल्कोहॉल तथा यौगिक I देता है जबकि K,KOH से अभिक्रिया करने पर केवल देता हैं
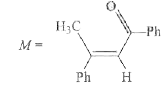
यौगिक H निम्न की अभिक्रिया से बनता है:
View Solutionएक तृतीयक ऐल्कोहॉल H अम्ल उत्प्रेरित निर्जलीकरण पर उत्पाद I देता है | I ओजोनी - अपघटन पर यौगिक J तथा K देता है | यौगिक J,KOH के साथ अभिक्रिया करने पर बेंजील ऐल्कोहॉल तथा यौगिक I देता है जबकि K,KOH से अभिक्रिया करने पर केवल देता हैं
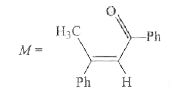
यौगिकों J,K तथा l की संरचनाएँ क्रमशः हैं
View SolutionA tertiary alcohol (H) upon acid-catalysed dehydration gives a product (I). Ozonolysis of (I) leads to compounds (J) and (K). Compound (J) upon reaction with KOH gives benzyl alcohol and a compound (L), whereas (K) on reaction with KOH gives only (M). Compound (H) is formed by the reaction of:
View SolutionA tertiary alcohol (H) upon acid-catalysed dehydration gives a product (I). Ozonolysis of (I) leads to compounds (J) and (K). Compound (J) upon reaction with KOH gives benzyl alcohol and a compound (L), whereas (K) on reaction with KOH gives only (M). The structures of compounds (J), (K), and (L), respectively, are:
View Solution

