Abhinav is a student of class IX. One day all the students were performing experiments in the science laboratory. Just then, the chemistry teacher, Mrs. Prasad, came to the laboratory. She had three beaker in her hands labelled A, B and C. All the beakers contained colourless liquids which looked exactly the same. Mrs. Prasad turned to the students and said that one of the beakers contained a pure substance whereas the other two beakers contained solutions of the same solute in the same solvent but different concentrations. She also told the students that the weight one of the three beakers A, B and C were 110g, 120g and 100g respectively. Mrs. Prasad wanted one of the students to find out experiementally which beaker contained a pure substance and which two beakers contained solutions. She also wanted them to show experimentally two properties which could tell why the solutions are considered mixtures, even though they are homogeneous substances. Mrs. Prasad wanted one of the the students to find out experimentally which beaker contained a pure substance and which two beakers contained solutions. She also wanted them to show experimentally two properties which could tell why the solutions are considered mixtures, even though they are homogeneous substances. Mrs. Prasad allowed them to use any required apparatus from the laboratory for the purpose. Abhinav offered to perform the experiements to distinguish between the pure substance and solutions and also to show experimentally why solutions are considered mixtures.
(a) What did Abhinav do to find out which of the given liquids is a pure substances and which liquids are solutions ? What were the observations made by Abhinav ?
(b) Whay did Abhinav conclude form the above observations ?
(c) Which property of the pure substance is exhibited by this experiment ?
(d) Which properties of a solution are exhibited in this experiement ?
(e) What values are displayed by Abhinav in this episode ?
(a) Abhinav took the three liquids A,B,C and D in three different china dishes and evaporated them by heating. Abhinav observed that :
(i) The liquid in beaker A evaporated evaporated leaving behind a white 'solid' residue.
(ii) The liquid in beaker B evaporated leaving behind much more quantity of white solid residue.
(iii) The liquid in beaker C evaporated completely, without leaving behind behind any residue
(b) Abhinav concluded that :
(i) Liquids A and B are solutions or mixtures (becuase they leave behind residue on evaporation)
(ii) Liquid C is a pure substance (because it evaporates completely, without leaving behind any residue)
(c) The property of a pure substance exhibited by this experiement is that is pure substance cannot be separated
(c) The property of a pure substance exhibited by this experiement is that a pure substance cannot be separated into its constituents by physical methods (here evaporation)
(d) The properties of a solution exhibited in this experiment are that :
(i) a solution can be separated into its components by physical methods (here evaporation)
(ii) a solution has a variable composition (here the two solutions contain different amounts of dissolved solids)
(e) The values displayed by Abhinav in this episode are (i) Knowledge, of pure substances and solution
(ii) Application of knowledge in solving problems, and
(iii) Boldness (to accept challenge)
Similar Questions
One day Vabhav was performing experiments in the science laboratory based on the of chemical combination. Just then his chemistry teacher, Mr. Rajeev, came into the laboratory. Mr. Rajeev told Vaibhav that in an experiment conducted byby a class IX student when 10.6 g of sodium carbonate was reacted with 12.0g of ethanoic acid in a closed flask, then 16.4 g of sodium ethanoate, 4.4 g of carbon dioxide and an unknown mass of substances Y were produced. Mr. Rajeev asked Vaibhav to make use of this information and answer the following questions : (a) Write a word equation for the reaction which takes place on reacting sodium carbonate and ethanoic acid (b) Which is the substance Y which is produced in this reaction ? (c) What mass of substance Y is produced in this reaction ? (d) Which law of chemical combination has been made use of in calculating the mass of substance Y ? State this law (e) What values are displayed by Vaibhav in this eposide ?
View Solutionजसप्रीत के पास एथनॉल और एयेनॉइक अम्ल दो बीकर में रखे ये किन्तु वह बीकरों पर यौगिकों का नाम लिखना भूल गयी। अब जसप्रीत कौन-सा प्रयोग करे कि पहचान सके कि किस बीकर में कौन-सा पदार्थ है ?
View SolutionThree beakers labelled as A, B and C each containing 25 mL of water were taken. A small amount of NaOH, anhydrous CuSO4 and NaCl were added to the beakers A, B and C respectively. It was observed that there was an increase in the temperature of the solutions contained in beakers A and B, whereas in case of beaker C, the temperature of the solution falls. Which one of the following statement(s) is (are) correct ?
(i) In beakers A and B, exothermic process has occurred.
(ii) In beakers A and B, endothermic process has occurred.
(iii) In beaker C exothermic process has occurred.
(iv) In beaker C endothermic process has occurred.
View SolutionThree beakers labelled as A, B and C each containing 25 mL of water were taken. A small amount of NaOH, anhydrous CuSO_(4) and NaCl were added to the beakers A, B and C respectively. It was observed that there was an increase in the temperature of the solutions contained in beakers A and B, whereas in case of beaker C, the temperature of the solution falls. Which one of the followig statement (s) is (are) correct? 1. In beakers A and B, exothermic process has occurred. 2. In beakers A and B, endothermic process has occurred. 3. In beaker C, exothermic process has occurred. 4. In beaker C, endothermic process has occurred.
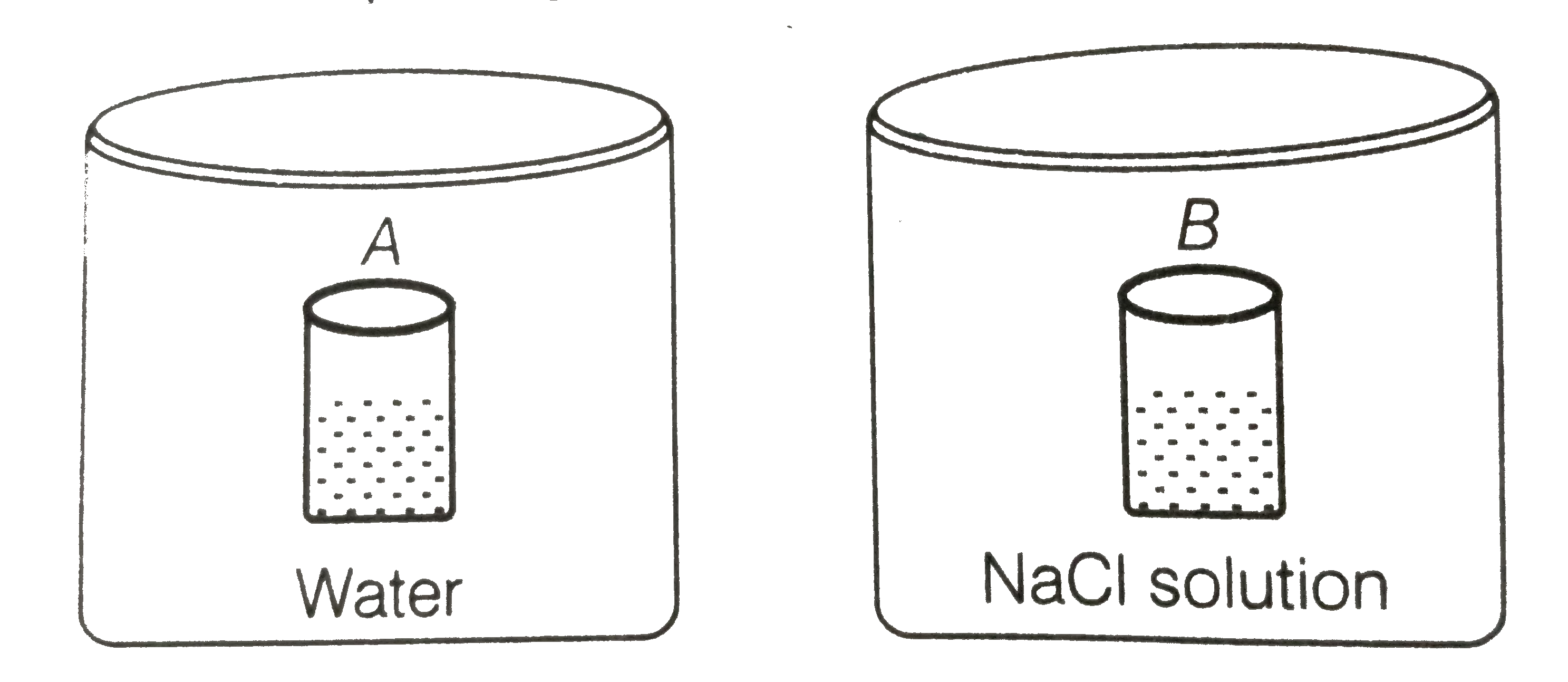
View SolutionTwo beakers of capacity 500 mL were taken. One of these beakers, labelled as "A" was filled with 400 mL water whereas the beaker labelled "B" was filled with 400 mL of 2M solution of NaCl. At the same temperature both the beakers ware placed in containers os same material and same capacity as shown in figure.
View SolutionA student prepared solutions of (i) an acid and (ii) a base in two separate beakers. She forgot to label the solutions and litmus paper is not available in the laboratory. Since both the solutions are colourless how will she distinguish between the two ?
View SolutionTwo beakers of capacity 500 mL were taken. One of these beakers, labelled as "A" was filled with 400 mL water whereas the beaker labelled "B" was filled with 400 mL of 2M solution of NaCl. At the same temperature both the beakers ware placed in containers os same material and same capacity as shown in figure.

View Solution"There are two beakers containing aqueous Kl solution.' A student adds little KMnO_4 alone the first beaker and KMnO_4 and sul-phuric acid to the second beaker:- Give the balanced chemical equation for the reaction in each beaker.
View Solution
