An electrolyte A gives 3 ions and B is a non-electrolyte. If 0.1 M solution of B produces an osmotic pressure P, then 0.05 M solution of A will produce an osmotic pressure, assuming that the electrolyte is completely ionised :
0.02p
0.8p
0.4p
0.6p
The correct Answer is:B
For two solutions .
π(A)π(B)=c(A)c(B)
c(B)=0.1M,c(A)=0.02×4=0.08M
π(A)p=0.080.1=0.8
∴π(A)=0.8p
Topper's Solved these Questions
- SOLUTIONSBook:MODERN PUBLICATIONChapter:SOLUTIONSExercise:COMPETITION FILE (MARTIX MATCH TYPE QUESTIONS)
Explore 2 Videos
- SOLUTIONSBook:MODERN PUBLICATIONChapter:SOLUTIONSExercise:COMPETITION FILE (INTEGER TYPE OR NUMERICAL VALUE TYPE QUESTIONS )
Explore 13 Videos
- SOLUTIONSBook:MODERN PUBLICATIONChapter:SOLUTIONSExercise:COMPETITION FILE (C. MULTIPLE CHOICE QUESTIONS(MCQ))
Explore 10 Videos
- SOLID STATEBook:MODERN PUBLICATIONChapter:SOLID STATEExercise:UNIT PRACTICE TEST
Explore 13 Videos
- SURFACE CHEMISTRYBook:MODERN PUBLICATIONChapter:SURFACE CHEMISTRYExercise:COMPETITION FILE (OBJECTIVE TYPE QUESTIONS) (MATCHING LIST TYPE QUESTIONS)
Explore 2 Videos
Similar Questions
Match the behaviour of solution in Column I with the example listed in Column II.

View SolutionThe liquid pair of acetone-chloroform shows a positive deviation form Raoult's law.
View SolutionColligative properties of a solution depends upon
View SolutionThe ratio of closed packed atoms to tetrahedral holes in cubic close packing is :
View SolutionMatch list I with list II and select the correct answer using the code :
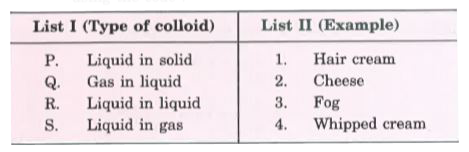
View SolutionSolute A is a ternary electrolyte and solute B is non-elctrolyte ,if0.1M solution of soluteB produces an osmotic pressure of 2P,then 0.05Msolution of Aat the same temperature will produce on osmotic pressure equal to
View SolutionThe solute A is a ternary electrolyte and solute B is a non- electrolyte. If 0.1 M solution of solute B produces an osmotic pressure of 2P, then, 0.05 M solution of A at the same temperature will produce an osmotic pressure equal to
View SolutionWhich of the following electrolytes have same osmotic pressure as that of 0.1 M KCl ?
View SolutionAn electrolyte A gives 3 ions and B is a non-electrolyte. If 0.1 M solution of B produces an osmotic pressure P, then 0.05 M solution of A will produce an osmotic pressure, assuming that the electrolyte is completely ionised :
View Solution1 molar solution of a non-volatile and non-electrolyte compound will produce an osmotic pressure ….. At 0∘C
View SolutionSolute A is a ternary electrolyte and solute B is a non-electrolyte. If 0.1 M solution of B produces an osmotic pressure of 2P at temperature 3T K then 0.05 M solution of A at T K will produce an osmotic pressure of:
View SolutionOsmotic pressure of a solution at 300 K is 73.8 atm. Assuming non-electrolytic solute, the molarity of solution will be :
View SolutionThe osmotic pressure (in atm.) of a 1 M solution a non-electrolyte at 0^(@)C will be
View SolutionThe osmotic pressure of 0.1 M aqueous solution of NaCl is ……………. Osmotic pressure of 0.1 M aqueous solution of glucose
View SolutionThe empirical formula of a non-electrolyte is CH2O. A solution containing 3 g L−1 of the compound exerts the same osmotic pressure as that of 0.05 M glucose solution. The molecular formula of the compound is :
View SolutionIn 0.1 M solution of a non electrolyte, ΨW value will be :
View Solution
