An element P (atomic number 20) reacts with an element Q (atomic number 17) to form a compound. Answer the following questions giving reason:
Write the position of P and Q in the Modern Periodic Table and the molecular formula of the compound formed when P reacts with Q.Solution in Kannada
change X to Q and Y to P in the above answer. Formula of the compound is PQ2
Similar Questions
An element X (atomic number 17) reacts with an element Y (atomic number 20) to form a compound.
(i) Write the position of these elements in the modern periodic table.
(ii) Write the formula of the compound formed. Justify your answer in each case.
View SolutionAn element X (atomic number 17) reacts with an element Y (atomic number 20) to form a Compound.
i]Write the position of these elements in the Modern Periodic Table
ii] Which one is a metal and which one is a non-metal?
iii] Write the formula of the compound.
View SolutionAn element X with atomic number 12 forms a compound with element Y with atomic number 17. The formula of the compound formed is
View SolutionAn element X with atomic number 12 forms a compound with element Y with atomic number 17. The formula of the compound formed is
View SolutionAn element P(atomic number 20) reacts with an element Q (atomic number 17) to form a compound. Answer the following questions giving reasons :
(a) Write the positions of P and Q in the Modern Periodic Table.
(b) Write the molecular formula of the compound when P reacts with Q.
View SolutionIn the following table, seven elements P,Q,R,S,T,U and V (here letters are not the usual symbol of the elements)of the modern periodic table with their atomic numbers are given
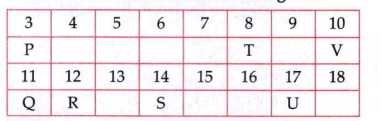 If Q combines with U, what would be the formula of the compound formed? If Q and U are replaced by their respective metals what will be the formula of the compound formed.
If Q combines with U, what would be the formula of the compound formed? If Q and U are replaced by their respective metals what will be the formula of the compound formed.
View SolutionA compound is formed by two elements P and Q. The element Q forms ccp and atoms of P occupy 1/3^rd ofthe tetrahedral voids. What is the formula of the compound?
View Solution
