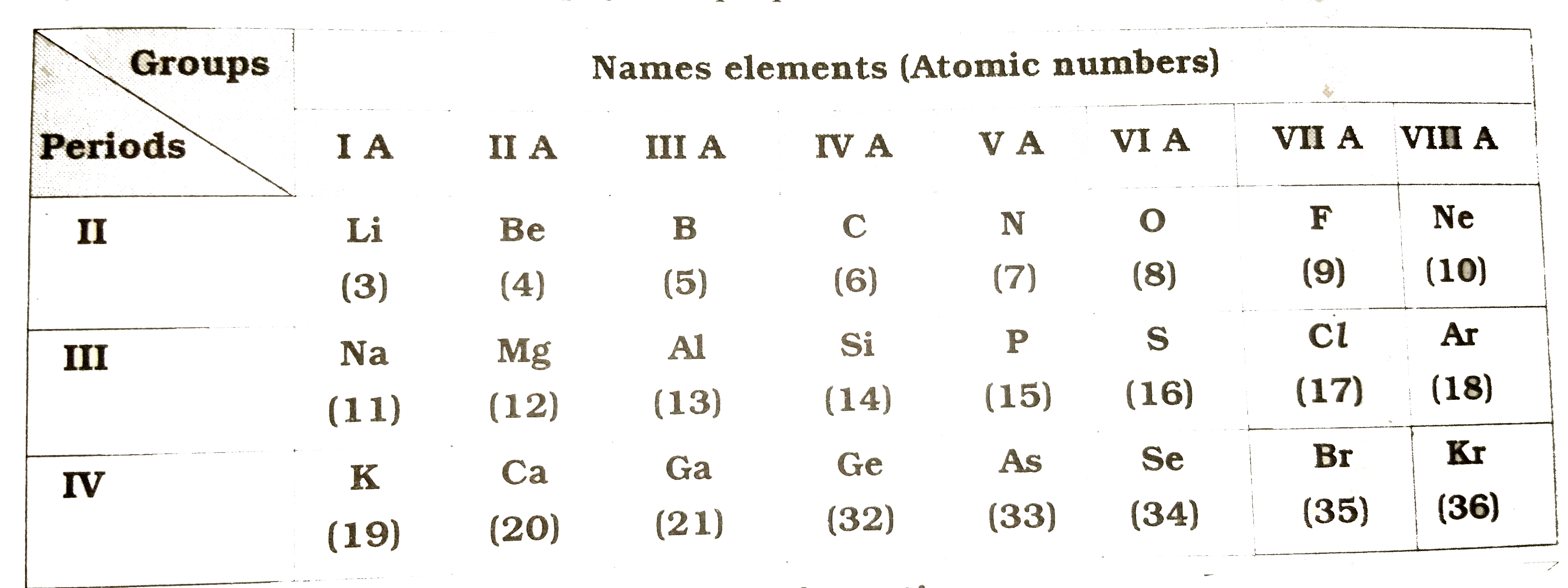
Answer the following from the above information .
Which element has electrons configuration of 1s22s22p4Solution in Telugu
Oxygen.
Similar Questions
Electronic configuration of element "A" is 1s^(2)2s^(2)2p^(4)" . Electronic configuration of element "B" is 1s^(2)2s^(2)2p^(6)3s^(2) . Formula of compound formed is
View Solutioniv) 1s22s22p4 ఎలక్ట్రాన్ విన్యాసం గల మూలకం ఏది?
View SolutionWhich element has electron configuration 1s22s22p2?
View SolutionWhich of the following element is represented by electronic configuration 1s2,2s2,2p1_x,2p1_y,2p1_z
View SolutionThe electronic configuration for the following atoms are given below : a. 1s^(2) 2s^(2) 2p^(5) , b. 1s^(2) 2s^(2) 2p^(4) , c. 1s^(2) 2s^(2) 2p^(6) 3s^(2) d. 1s^(2)2s^(2) 2p^(6) , e. 1s^(2)2s^(2)2p^(6) 3s^(1) i. From the above configuration, arrange them in decreasing IE . ii. Which of the electronic configuration given above wil have the lowest IE ? iii. Which of the electronic configuration given above will be for noble gases ?
View SolutionTo which element does the following electronic configuration correspond ?
1s22s22p2
View SolutionThe element whose electronic configuration is 1s22s22p5 is
View SolutionWhich element has electronic configuration of 1s^2 2s^2 2p^6 ?
View Solution
