Arrange the following:
Elements of group 17, in decreasing order of reactivity.
To arrange the elements of Group 17 (the halogens) in decreasing order of reactivity, we can follow these steps:
1. Identify the Elements in Group 17: The elements in Group 17 are Fluorine (F), Chlorine (Cl), Bromine (Br), and Iodine (I).
2. Understand Reactivity Trends: In Group 17, the reactivity of the halogens decreases as you move down the group. This means that the element at the top of the group is more reactive than those below it.
3. List the Elements from Top to Bottom: The order of the elements from top to bottom in Group 17 is:
- Fluorine (F)
- Chlorine (Cl)
- Bromine (Br)
- Iodine (I)
4. Arrange in Decreasing Order of Reactivity: Based on the trend that reactivity decreases down the group, we can arrange the elements in decreasing order of reactivity as follows:
- Fluorine (F) - most reactive
- Chlorine (Cl)
- Bromine (Br)
- Iodine (I) - least reactive
Final Arrangement:
The decreasing order of reactivity for the elements of Group 17 is:
Fluorine (F) > Chlorine (Cl) > Bromine (Br) > Iodine (I)
---
- Fluorine (F)
- Chlorine (Cl)
- Bromine (Br)
- Iodine (I)
- Fluorine (F) - most reactive
- Chlorine (Cl)
- Bromine (Br)
- Iodine (I) - least reactive
The decreasing order of reactivity for the elements of Group 17 is:
Fluorine (F) > Chlorine (Cl) > Bromine (Br) > Iodine (I)
Topper's Solved these Questions
- THE PERIODIC TABLE BOOK - ICSECHAPTER - THE PERIODIC TABLE EXERCISE - TOPIC 1 Modern Periodic Table and its Periodicity (1 Mark Questions )
25 Videos
- THE PERIODIC TABLE BOOK - ICSECHAPTER - THE PERIODIC TABLE EXERCISE - TOPIC 1 Modern Periodic Table and its Periodicity (2 Marks Questions )
50 Videos
- THE PERIODIC TABLE BOOK - ICSECHAPTER - THE PERIODIC TABLE EXERCISE - TOPIC 2 Studying Individual Groups (5 Marks Questions)
34 Videos
- THE LANGUAGE OF CHEMISTRYBOOK - ICSECHAPTER - THE LANGUAGE OF CHEMISTRYEXERCISE - EXCERCISE 1(C ) (Correct the following statement)
21 Videos
- WATERBOOK - ICSECHAPTER - WATEREXERCISE - TOPIC 2 Solution (5 MARKS QUESTIONS)
5 Videos
Similar Questions
The longest period of periodic table has ____ elements [36/32]
View SolutionWhy did Mendeleev left gaps in his periodic table ?
View SolutionName the alkali metals. How many electrons(s) do they have in their outermost orbit.
View SolutionCorrect the statement : A molecular formula represents an element.
View SolutionNormally, solubility of a crystalline solid increases with temperature. Does it increase uniformly in all cases ? Name a substance whose solubility :
increases gradually with temperature.
View SolutionThe increasing order of reactivity among group 1 elements is LiltNaltKltRbltCs whereas that among group 17 elements is FgtClgtBrgtI . Explain.
View SolutionArrange the following in decreasing order of their reactivity with an electrophile.
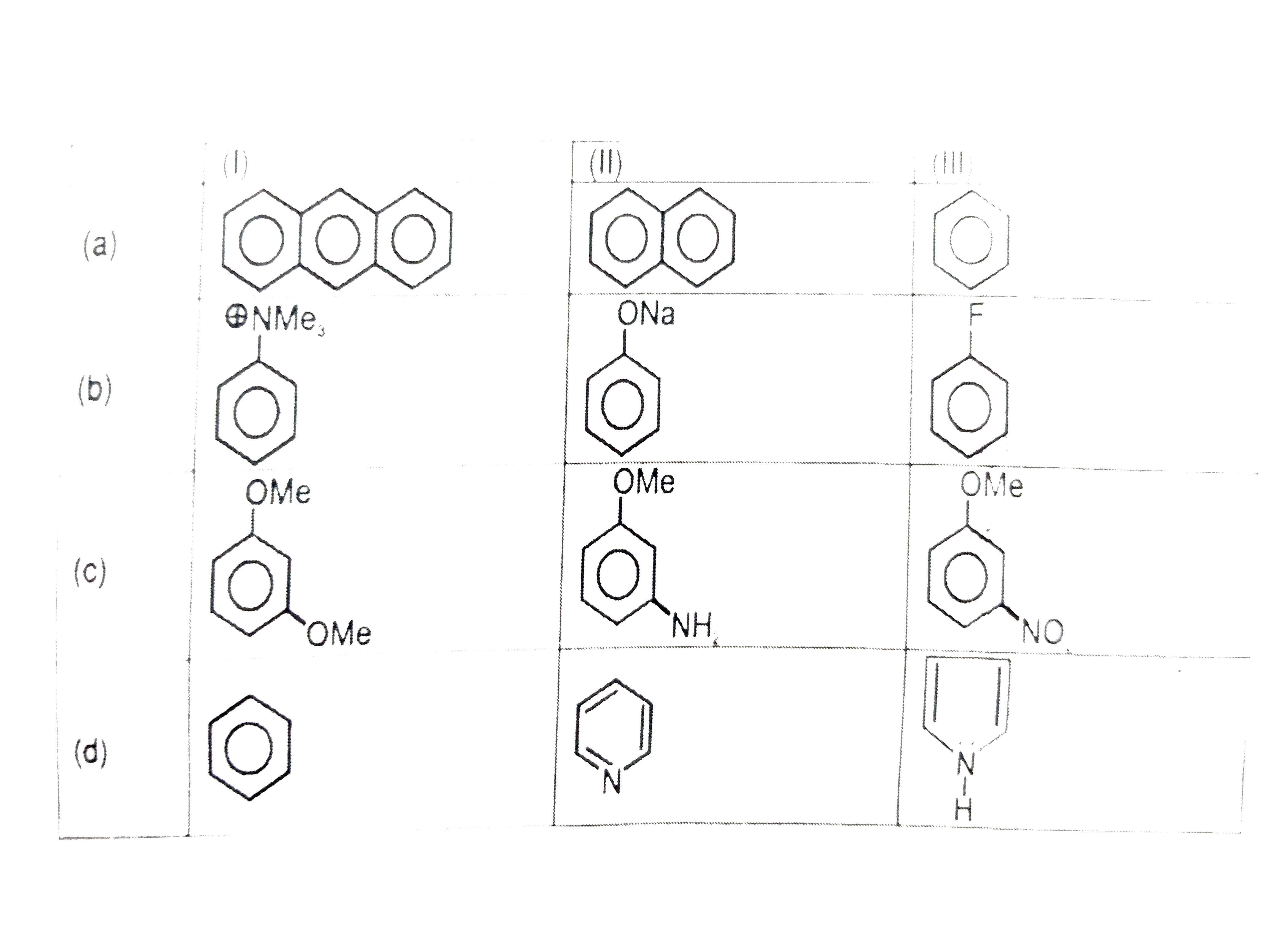
View Solutionप्रथम वर्ग के तत्वों के लिए अभिक्रियाशीलता का बढ़ता क्रम इस प्रकार है-
Li<Na<K<Rb<Cs जबकि वर्ग 17 के तत्वों का क्रम F>Cl>Br>I है। इसकी व्याख्या कीजिए।
View Solutionनिम्न क्रियात्मक समूहों की अभिक्रियाशीलता के घटने का क्रम होगा-
View Solutionवर्ग-17 के तत्वों को उनकी आयनन ऐन्थेल्पी तथा विद्युत् ऋणात्मकता के घटते क्रम में व्यवस्थित कीजिए।
View SolutionThe increasing order of reactivity among group 1 elements is LiltNaltKltRbltCs whereas that among group 17 elements is FgtClgtBrgtI . Explain.
View SolutionThe least reactive element in group 17 is
View SolutionFor hydrolysis of the following functional groups, the decreasing order of reactivity
View SolutionFor hydrolysis of the following functional groups,the decreasing order of reactivity is:
View SolutionFor hydrolysis of the following functional groups,the decreasing order of reactivity is:
View Solution
