Assertion (A): In paper chromatography, the compound is identify on the base of Rf value.
Reason (R ): The Rf value of every compound is definate and constant
Statement (A) and Reason (R ) both are right. Statement (R ) is correct explanation of statement (A)
Statement (A) and Reason (R ) both are right, but (R ) is not correct explanation of statement (A)
Both Statement (A) and (R ) are wrong
Statement (A) is right but statement (R ) is wrong
The correct Answer is:A
Rf is retardation factor
Rf=Distance moved by the substance from base lineDistance moved by the solvent from base line
Rf vlaue of every compound is definite and constant like boiling point, melting point, density etc. So, on the base of Rf value to get the name of compound
Topper's Solved these Questions
- ORGANIC CHEMISTRY SOME BASIC PRINCIPLES AND TECHNIQUESBook:KUMAR PRAKASHANChapter:ORGANIC CHEMISTRY SOME BASIC PRINCIPLES AND TECHNIQUESExercise:Section -C (MCQs From Testual Exercise)
Explore 10 Videos
- ORGANIC CHEMISTRY SOME BASIC PRINCIPLES AND TECHNIQUESBook:KUMAR PRAKASHANChapter:ORGANIC CHEMISTRY SOME BASIC PRINCIPLES AND TECHNIQUESExercise:Section -C - Darpan.s Examination Oriented MCQs
Explore 206 Videos
- ORGANIC CHEMISTRY SOME BASIC PRINCIPLES AND TECHNIQUESBook:KUMAR PRAKASHANChapter:ORGANIC CHEMISTRY SOME BASIC PRINCIPLES AND TECHNIQUESExercise:Section-B - Fill in the Blanks
Explore 92 Videos
- HYDROGENBook:KUMAR PRAKASHANChapter:HYDROGENExercise:Section-D NCERT Exemplar (Long Answer)
Explore 8 Videos
- QUESTION ASKED IN JEE -2018Book:KUMAR PRAKASHANChapter:QUESTION ASKED IN JEE -2018Exercise:MCQ
Explore 10 Videos
Similar Questions
In the organic compound CH2=CH−CH2−CH2−≡CH, the pair of hydridised orbitals involved in the formation of C2−C3 bond is
View SolutionHow the functional group can be entered in organic compound?
View SolutionGenetic information containing molecules is known as…..
View SolutionAtomic hydrogen combines with almost all elements but molecular hydrogen does not. Explain.
View SolutionAccording to molecular orbital theory, which of the following will not be a viable molecule?
View SolutionAssertion : Moving phase is liquid and stationary phase is solid in paper chromatography. Reason : Paper chromatography is used for analysis of polar organic compounds.
View SolutionTwo stereoisomers (Shown in fischer projection ) from an epoxide, when treated with base. r_(A) and r_(B) are rate of epoxide formation for compound (A) and compound(B) with base. Identify the correct option?
View SolutionA substance is analyzed by paper chromatography, giving the chromatogram shown.
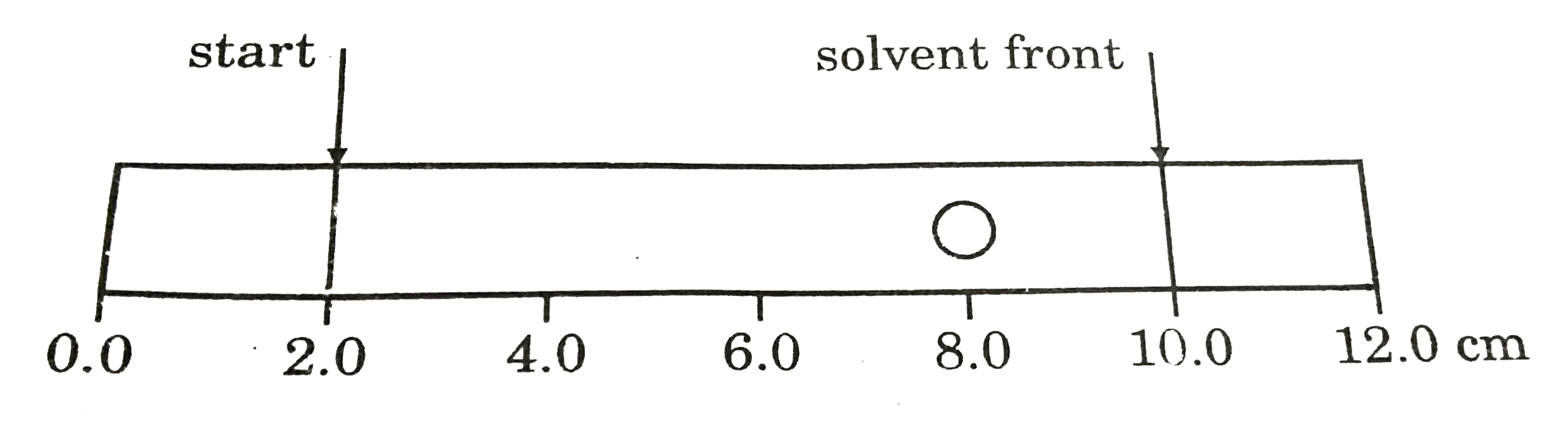
What is the Rf value of the substance represented by the spot at 8.0 cm?
View SolutionAssertion : Moving phase is liquid and stationary phase is solid in paper chromatography.
Reason : Paper chromatography is used for analysis of polar organic compounds.
View SolutionExplain the principles of adsorption chromatography and partition chromatography. Define R_(f) value. What is called descending paper chromatography?
View SolutionIn chromatography, which of the following statements is INCORRECT for Rf ?
View SolutionWhat is the characteristic of Rf value ?
View SolutionIn TLC for compound X Rf=0.7 and for B Rf=0.4 which substance migrate more?
View SolutionAssertion (A): Paper chromatography is a type of partition chromatography. Reason (R ): In it paper is as a stationary phase and solute is as a mobile phase
View SolutionWhat is the characteristic of R_(f) value ?
View Solution

