Assertion: Elevation in boiling point is a colligative property.
Reason: Elevation in boiling point is directly proportional to molarity.
If both assertion and reason are true and reason is the correct explanation of assertion.
If both assertion and reason are true, but reason is not the correct explanation of assertion.
If assertion is true, but reason is false.
If both assertion and reason are false
The correct Answer is:C
When a solute is dissolved in a solvent the boiling point of a solvent will increase. This is referred to as boiling point elevation. This elevation in boiling point is directly dependent on the amount of solute present in the solution but it is not based on the nature of the solute, hence, elevation in boiling point is considered as colligative property. However, elevation in boiling point is directly proportional to molality of the solution not molarity. Thus, assertion is true but reason is false.s
Topper's Solved these Questions
- SOLUTIONSBOOK - ICSECHAPTER - SOLUTIONSEXERCISE - MULTIPLE CHOICE QUESTIONS (Structure based questions)
4 Videos
- SOLID STATEBOOK - ICSECHAPTER - SOLID STATEEXERCISE - ISC EXAMINATION QUESTIONS PART-I (Numerical Problems)
6 Videos
- SOME IMPORTANT ORGANIC NAME REACTIONS BOOK - ICSECHAPTER - SOME IMPORTANT ORGANIC NAME REACTIONS EXERCISE - Questions
278 Videos
Similar Questions
Knowledge Check
Colligative properties like osmotic pressure, elevation in boiling point etc. depend upon
Anumber of solute particles and polarity of solventBon the degree of polarity of soluteConly on the number of solute particlesDnumber of solute particles and the nature of solute.
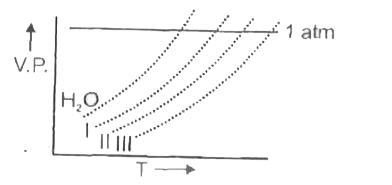
Which is having highest elevation in boiling point?
View SolutionConsider following figure and answer the questions at the end of it. Figure explains elevation in boiling point when a non-volatile solute is added to a solvent.
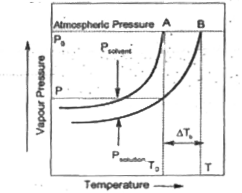
Variation of vapour pressure with temperature and showing elevation in boiling point.
Given that ΔTb is the elevation in boiling point of the solvent in a solution of molality m then Ltm→0(ΔTbm) is equal to
View SolutionBoiling point elevation is
View SolutionThe molal elevation constant is the ratio of the elevation in boiling point to :
View SolutionAssertion (A): The elevation in boiling point for two isotonic solutions may not be same.
Reason (R ): The boiling point depends upon the concentration of the solute.
View SolutionWhich solution will show maximum elevation in boiling point?
View SolutionEach question contains STATEMENT-I(Assertion) and STATEMENT-2(Reason).the statement carefully and mark the correct answer according to the instruction given below:
STATEMENT - 1 : The boiling point of 0.1 M urea solution is less than that of 0.1 M KClsolution.
STATEMENT - 2 : Elevation of boiling point is directly proportional to the number of moles of non-volatile solute particles present in the solution.
View Solution

