At constant temperature, on the basis of given graph which gas possess higher solubility?
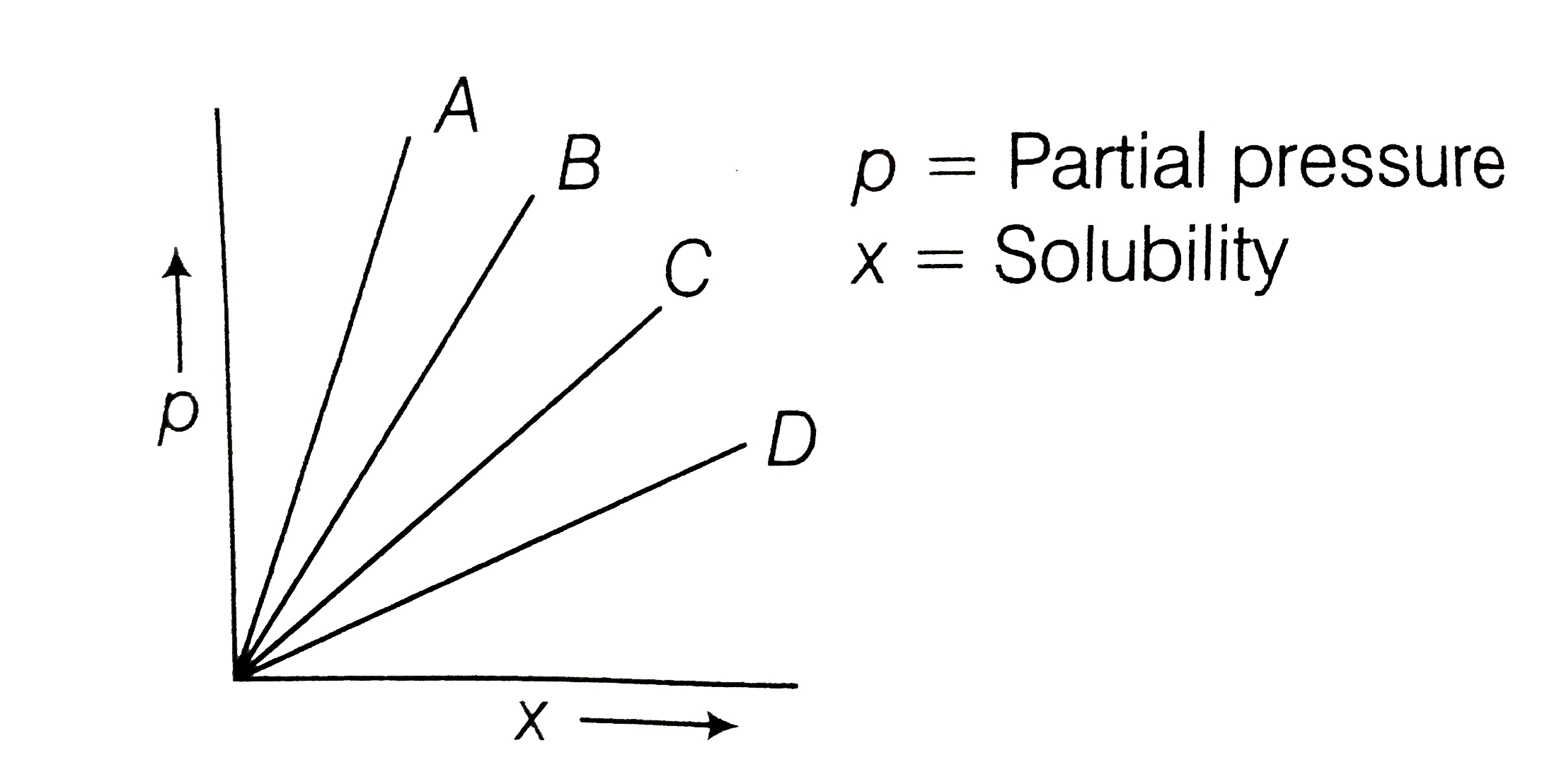
A
B
C
D
The correct Answer is:D
Slope= KH
Solubility of gas is more which have less value of KH
∴ Gas D has more solubility.
Topper's Solved these Questions
Similar Questions
At constant temperature, in a given mass of a gas.
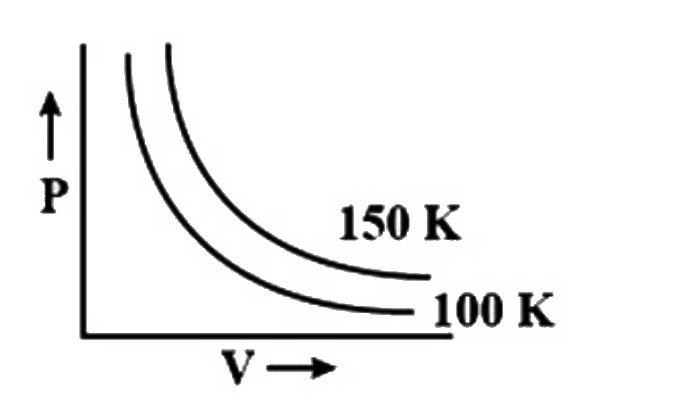
View SolutionA graph is plotted between pressure and volume at different temperature. On the basis of the graph what changes will you observe in the volume if
(i) the pressure is increased at constant temperature
(ii) the temperature is decreased at constant pressure
View Solution
Knowledge Check
At constant temperature, in a given mass of an ideal gas -
AThe ratio of pressure and volume always remains constantBVolume always remains constantCPressure always remains constantDThe product of pressure and volume always remains constantAt constant temperature, in a given mass of an ideal gas -
AThe ratio of pressure and volume always remains constantBVolume always remains constantCPressure always remains constantDThe product of pressure and volume always remains constantAt constant temperature, in a given mass of an ideal gas -
AThe ratio of pressure and volume always remains constantBVolume always remains constantCpressure always remains constantDThe product of pressure and volume always rmains constant
At constant temperature, the rate of tranpiration will be higher at
View SolutionAt constant temperature, the rate of transpiration will be higher at
View SolutionAt a constant temperature, the amount of a given gas that dissolves in a liquid is directly proportional to the partial pressure of that gas in equilibrium with that liquid. Which law is this ?
View SolutionIf heat energy is given to an ideal gas at constant pressure, then select the graph which best represents the variation of VT with temperature (T).
View SolutionAt a constant pressure, of the following graphs that one which represents the variation of the density of an ideal gas with the absolute temperature T, is
View Solution
