By which of the following reagents can a sublimate of HgCl2 be distinguished from NH4Cl ?
H2S
BaCl2
NaNO3
FeCl3
The correct Answer is:A
To distinguish between a sublimate of HgCl₂ (mercury(II) chloride) and NH₄Cl (ammonium chloride), we can follow these steps:
1. Identify the Compounds:
- We have two substances: HgCl₂ (mercury(II) chloride) and NH₄Cl (ammonium chloride).
2. Consider the Reagents:
- The reagents provided for the distinction are H₂S (hydrogen sulfide), barium chloride, sodium nitrate, and ferric chloride.
3. Test with H₂S:
- When H₂S is added to HgCl₂, a black precipitate of HgS (mercury sulfide) is formed, and hydrochloric acid (HCl) is released.
- The reaction can be represented as:
HgCl2+H2S→HgS (black ppt)+2HCl
- In contrast, NH₄Cl does not react with H₂S, meaning no precipitate will form.
4. Conclusion from H₂S Test:
- Since HgCl₂ produces a black precipitate with H₂S while NH₄Cl does not react, we can distinguish between the two substances using H₂S.
5. Test with Other Reagents:
- Barium Chloride: This reagent does not react with either HgCl₂ or NH₄Cl in a way that would allow for distinction.
- Sodium Nitrate: Similarly, sodium nitrate does not provide a distinguishing reaction with either compound.
- Ferric Chloride: Ferric chloride also does not react with either HgCl₂ or NH₄Cl to create a distinguishing characteristic.
6. Final Answer:
- The only reagent that allows us to distinguish between HgCl₂ and NH₄Cl is H₂S.
Final Answer:
The reagent that can be used to distinguish a sublimate of HgCl₂ from NH₄Cl is H₂S (hydrogen sulfide).
- We have two substances: HgCl₂ (mercury(II) chloride) and NH₄Cl (ammonium chloride).
2. Consider the Reagents:
- The reagents provided for the distinction are H₂S (hydrogen sulfide), barium chloride, sodium nitrate, and ferric chloride.
- When H₂S is added to HgCl₂, a black precipitate of HgS (mercury sulfide) is formed, and hydrochloric acid (HCl) is released.
- The reaction can be represented as:
HgCl2+H2S→HgS (black ppt)+2HCl
- In contrast, NH₄Cl does not react with H₂S, meaning no precipitate will form.
- Since HgCl₂ produces a black precipitate with H₂S while NH₄Cl does not react, we can distinguish between the two substances using H₂S.
- Barium Chloride: This reagent does not react with either HgCl₂ or NH₄Cl in a way that would allow for distinction.
- Sodium Nitrate: Similarly, sodium nitrate does not provide a distinguishing reaction with either compound.
- Ferric Chloride: Ferric chloride also does not react with either HgCl₂ or NH₄Cl to create a distinguishing characteristic.
- The only reagent that allows us to distinguish between HgCl₂ and NH₄Cl is H₂S.
The reagent that can be used to distinguish a sublimate of HgCl₂ from NH₄Cl is H₂S (hydrogen sulfide).
Topper's Solved these Questions
- QUALITATIVE INORGANIC ANALYSISBook:VK JAISWAL ENGLISHChapter:QUALITATIVE INORGANIC ANALYSISExercise:MATCH THE COLUMN
Explore 3 Videos
- QUALITATIVE INORGANIC ANALYSISBook:VK JAISWAL ENGLISHChapter:QUALITATIVE INORGANIC ANALYSISExercise:ASSERTION-REASON TYPE QUESTIONS
Explore 13 Videos
- QUALITATIVE INORGANIC ANALYSISBook:VK JAISWAL ENGLISHChapter:QUALITATIVE INORGANIC ANALYSISExercise:LEVEL 3
Explore 44 Videos
- PERIODIC PROPERTIESBook:VK JAISWAL ENGLISHChapter:PERIODIC PROPERTIESExercise:MATCH THE COLUMN
Explore 11 Videos
- s-BLOCK ELEMENTSBook:VK JAISWAL ENGLISHChapter:s-BLOCK ELEMENTSExercise:SUBJECTIVE PROBLEMS
Explore 2 Videos
Similar Questions
Match the following columns

View SolutionAssertion: AgNO3 reacts with KCN to form white ppt. of AgCN. This white ppt. Disappears when excess KCN is added.
Reason: AgCN decomposes to form silver-carbide and evolve N2 gas.
View SolutionA colourless inorganic compound (A) imparts a green colour to the flame. Its solution gives a white ppt. (B) with H2SO4. When heated with K2Cr2O7 and conc. H2SO4, a brown red vapour/gas (C) is formed. The gas/ vapour when passed through aqueous NaOH solution, it turns into a yellow solution (D) which forms yellow precipitate (E) with CH3COOH and (CH3COO)2Pb. with reference to above information, answe the following questions.
Q. The colourless inorganic compound (A) is:
View Solution
View Solution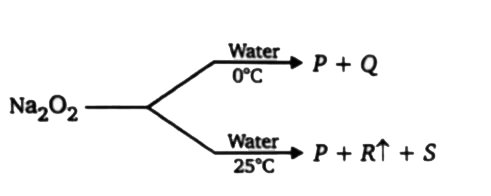
Calculate sum of bond order between same bonded atoms in Q and R compounds.
View SolutionThe reagents NH4Cl and NH3 will precipitate :
View SolutionWhich of the following reagents can distinguish methanol from ethanol ?
View SolutionThe reagents, NH4Cl and aqueous NH3 will precipitate
View SolutionCorrosive sublimate (HgCl2) can be used to distinguish between
View SolutionDistinguish sublimate from sublime.
View SolutionThe reagents, NH4Cl and aqueous NH3 will precipitate
View SolutionThe reagent, NH_(4)Cl and aqueous NH_(3) will precipitate
View SolutionWhich of the following reagents can be used to distinguish phenol from Anisole ?
View SolutionWhich of the following reagents can be used to distinguish Benzaldehdyde from acetophenone?
View SolutionCorrosive sublimate (HgCl_(2)) can be used to distinguish between
View SolutionCorrosive sublimate, HgCl2 can be used to distinguish between
View Solution
