Calculate the atomic mass (average) of chlorine using the following data:
% natural abundanceMolar mass.35Cl75.7734.9689.37Cl24.2336.9659
It is given two isotopes of bromine are 79/35Br(49.7%) and 81/35Br(50.3%). Then, the average atomic mass of bromine atom is given by
79×49.7100+81×50.3100
=3926.3100+4074.3100
8000.6100
=80.006u
Similar Questions
Knowledge Check
Average atomic mass of chlorine is 35.5 then the correct naturally occuring molar ratio of 35Cl&37Cl is
A4:1B2:1C3:1D1:1Use the data given in the following table to calculate the molar mass of naturally occurring agfon.
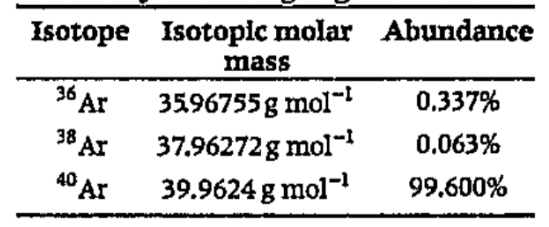
The molar mass of argon isA39.948B39.665C38.678D38.442
Calculate the average atomic mass of hydrogen using the following data :
Isotope% Natural abudance massAtomic mass(amu).1H 99.985 1.2H 0.015 2
View SolutionCalculate the average atomic mass of hydrogen using the following data
Isotope % Natural abundance Molar mass
.1H99.9851
.2H0.0152
View SolutionCalculate the average atomic mass of hydrogen using the following data :
Isotope% Natural abudance massAtomic mass(amu).1H 99.985 1.2H 0.015 2
View SolutionCalculate the atomic mass of chlorine, which consists of 76% of all the chlorine in nature, and chlorine - 37 that makes up the other 24%.
View SolutionUse data given in the following table to calculate the molar mass of naturaly occuring argo isotopes:
IsotopeIsotopic molar massAbundance.36Ar35.96755gmol−10.337%.38Ar37.96272gmol−10.063%.40Ar39.9624gmol−199.600%
View SolutionUse data given in the following table to calculate the molar mass of naturaly occuring argon isotopes:
IsotopeIsotopic molar massAbundance.36Ar35.96755gmol−10.337%.38Ar37.96272gmol−10.063%.40Ar39.9624gmol−199.600%
View Solution