Classify the elements based on their property of conductivity with suitable examples.
Text Solution
Elements can be classified into three categories on the basis of their conductivity:
(1) Metallic elements : They have high values of electrical conductivity. For example, Copper (Cu), Aluminium (Al). Silver (Ag).
(2) Non-metallic elements : They have low values of electrical conductivity. For example, Oxygen (O2). Nitrogen (N2). Chlorine (Cl2).
(3) Metalloids : The values of their electrical conductivity is lower than metals but higher than non-metals. For example, Silicon (Si), Germanium (Ge).
|
Similar Questions
Knowledge Check
Who classified elements based on atomic numbers ?
ANewlandsBMendeleevCDobereinerDMoseleyWho classified elements based on atomic numbers?
ANewlandsBMendeleevCDobereinerDMoseleyHitesh has classified some common items based on their properties as shown in the table .
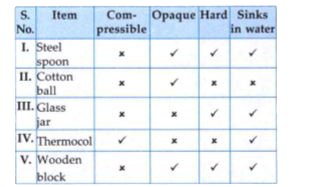
Which of the given items are not correctly classified ?AII and III onlyBI and IV onlyCII,IV and V onlyDNone of these
What are pisces ? Classify and explain two different kinds of fishes by the help of suitable examples.
View SolutionHow ligands are classified ? Explain with suitable examples.
View SolutionWho classified the elements on the basis of fundamental properties of elements like atomic mass?
View SolutionWho classified the elements on the basis of fundamental properties of elements like atomic mass?
View SolutionClassify the chemical reactions into various types types of valencies of cations with examples. depending on the way the products are formed by giving suitable examples.
View Solution
