Compare the stability of Ni4+ and Pr4+ from their ionisation enthalpy values.
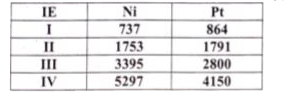
Pt4+ compounds are stable than Ni4+ compounds because the energy needed to remove 4 electrons in Pt is less than that of Ni
Topper's Solved these Questions
- TRANSITION AND INNER TRANSITION ELEMENTSBook:FULL MARKSChapter:TRANSITION AND INNER TRANSITION ELEMENTSExercise:Additional Questions (I. Choose the correct answer)
Explore 47 Videos
- TRANSITION AND INNER TRANSITION ELEMENTSBook:FULL MARKSChapter:TRANSITION AND INNER TRANSITION ELEMENTSExercise:Additional Questions (II. Fill in the blanks)
Explore 31 Videos
- TRANSITION AND INNER TRANSITION ELEMENTSBook:FULL MARKSChapter:TRANSITION AND INNER TRANSITION ELEMENTSExercise:Additional Questions (X. 5 Mark Questions.)
Explore 4 Videos
- SURFACE CHEMISTRYBook:FULL MARKSChapter:SURFACE CHEMISTRYExercise:ADDITIONAL QUESTIONS (5 Marks questions)
Explore 12 Videos
Similar Questions
Knowledge Check
Which of the following has abnormally low value of third ionisation enthalpy ?
AlanthanumBGadolimiumCLuteiumDAll of these
The sum of the first and second ionisation enthalpies and those of third and fourth ionisation enthalpies of nickel and platinum are given below:
IE1+IE2(kJmol−1)IE3+IE4(kJmol−1)Ni2.498.80Pt2.666.70
Taking these values into account write :
(i) the most common oxidation state for Ni and Pt and its reason.
(ii) the name of the metal (Ni or Pt) which can form compounds in +4 oxidation state more easily and why?
View SolutionWhich out of Li or Na has greater value for the following properties :
(i) Hydration ethalpy (ii) Stability of hydride
(iii) Stability of carbonate (iv) Basic character of hydroxide
(v) Ionisation enthalpy .
View SolutionCompare the alkali metals and alkaline earth metals with respect to (a) ionisation enthalpy, (b) basicity of oxides and (c ) solubility of hydroxides.
View SolutionCompare the alkali metals and alkaline earth metals with respect to (a) ionisation enthalpy, (b) basicity of oxides and (c ) solubility of hydroxides.
View SolutionThe energy required to remove an electron from an isolated gaseous atom in its ground state is called ionisation enthalpy.From the following, select the element having maximum value of 2nd ionization enthalpy.
Be
C
N
O
View SolutionCompare the first and second ionisation enthalpies of sodium and magnesium .
View SolutionCompare the general trend in the following properties of the elements of group 13 and 14 :
Ionisation enthalpy,
View Solution

