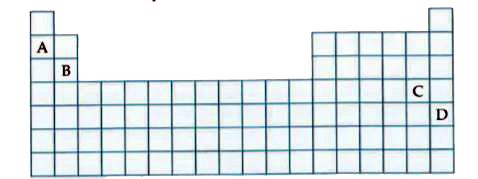
Consider the following elements, A, B, C and Din the given periodic table :

Which element forms acidic oxides?
Element B and C forms acidic oxides.
Similar Questions
An element .X. is forming an acidic oxide. Its position in modern Periodic Table will be
View SolutionThe positions of four elements A, B, C and D in the Modern Periodic Table are shown below. Which element is most likely to form an acidic oxide ?
View SolutionName or state with reference to the elements of the modern periodic table.
The element in period-3 which does not form an oxide.
View SolutionName the element with reference to the elements of the modern periodic table.
The element in the period 3 which does not form an oxide.
View Solutionआवर्त सारणी के किसी आवर्त में बाएँ से दाएँ जाने पर तत्त्वों के ऑक्साइड का अम्लीय गुण
View SolutionAmong the second period elements, the most acidic oxide is formed by the element of group:
View SolutionAn element placed in Group 14 and 2nd period of the Periodic Table, burns in the presence of oxygen to form an acidic oxide. Identify the element.
View SolutionAn element placed in Group 14 and 2nd period of the Periodic Table, burns in the presence of oxygen to form an acidic oxide. State the electronic configuration and atomic number of the element.
View Solution
