Consider the part of periodic table given below and answer the following questions:

(i) State the most reactive metal.
(ii) How many shells does element d have ?
(iii) Identify the element having valency 2.
(iv) Write the number of electrons in the valence shell of j.
(v) Out of h and i, which element is more non-metallic in nature ?
(vi) Out of e and h, which element possess large atomic size?
(i) d (ii) 4 (iii) e and g (iv) 2 (v) (vi) e
Topper's Solved these Questions
- PERIODIC CLASSIFICATION OF ELEMENTSBOOK - KUMAR PRAKASHANCHAPTER - PERIODIC CLASSIFICATION OF ELEMENTSEXERCISE - ADDITIONAL QUESTIONS AND ANSWER ( Distinguish between the following: )
7 Videos
- PERIODIC CLASSIFICATION OF ELEMENTSBOOK - KUMAR PRAKASHANCHAPTER - PERIODIC CLASSIFICATION OF ELEMENTSEXERCISE - OBJECTIVE QUESTIONS AND ANSWERS ( Answer the following questions in one word :)
25 Videos
- PERIODIC CLASSIFICATION OF ELEMENTSBOOK - KUMAR PRAKASHANCHAPTER - PERIODIC CLASSIFICATION OF ELEMENTSEXERCISE - TEXTUAL EXERCISE
13 Videos
- MODEL QUESTION PAPER 01BOOK - KUMAR PRAKASHANCHAPTER - MODEL QUESTION PAPER 01EXERCISE - (SECTION-A) (MCQs)
1 Video
- QUESTION PAPER-2 (MARCH,2020 - BOARD'S QUESTIONS PAPER)BOOK - KUMAR PRAKASHANCHAPTER - QUESTION PAPER-2 (MARCH,2020 - BOARD'S QUESTIONS PAPER)EXERCISE - Section - D (Answer the following questions)
3 Videos
Similar Questions
Elements of a group and Elements of a period
View SolutionHow many elements are known till date?
View SolutionIn the modern periodic table, which are the metals among the first ten elements ?
View SolutionWhich scientist proposed the .Law of Triad. for the classification of elements ?
View SolutionName the chemical compound commonly used by your mother in the kitchen for making tasty crispy pakoras or cake. State its chemical formula and write the chemical equation of its production.
View SolutionThe electronic configuration of an element X is: K L M 2,8,6 (i) What is the group number of element X in the periodic table? (ii) What is the period number of element X in the periodic table? (iii) What is the number of valence electrons in an atom is X ? (iv) What is the valence of X ? (v) Is it a metal or a non metal?
View SolutionAn element A belongs to third period and group 1 of the periodic table. Find out: (i) the number of valence electrons in its atoms (ii) valency of the element (iii) metal or non-metal (iv) name of the element (v) name of the family to which this element belongs
View SolutionThe following diagram shows a part of the periodic table in which the elements are arranged according to their atomic numbers. ( The letters given here are not the chemical symbols of the elements):
(i) Which elements has a bigger atom , a or f ?
(ii) Which element has a higer valency, K or o ?
(iii) Which element is more metallic, i or K ?
(iv) Which element is more non - metallic , d or g ?
(v) Select a letter which represents a non-metal of valency 2.
(vi) Select a letter which represents a noble gas.
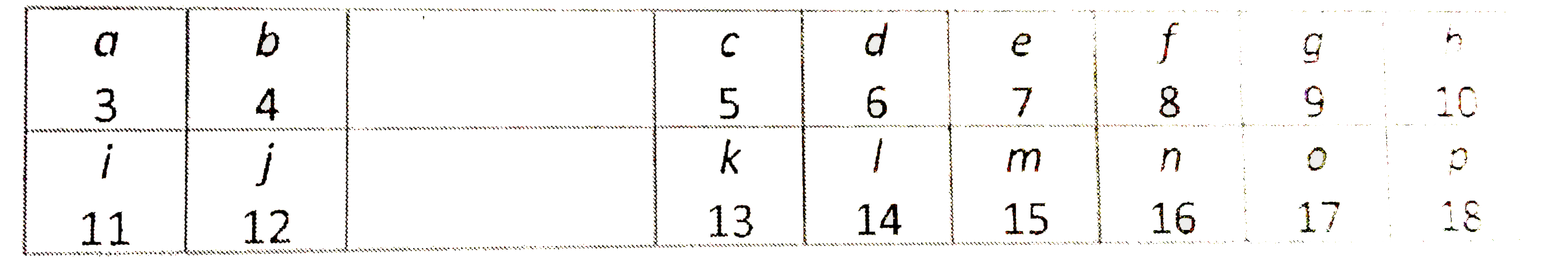
View SolutionThe atomic number of an element is 19.
(a) Write the electronic configuration of the element and determine (i) the valency of the element and (ii) whether the element is a metal or non-metal.
(b) Write the formula of the oxide of the element.
(c) Is the element more reactive or less reactive than Na (atomic number 11). Justify your answer giving example.
View SolutionHow do the following properties of elements change in a group of the periodic table ? (i) size of atoms (ii) Valency (iii) Ionization energy (iv) Electron affinity (v) Metallic character (vi) Chemical reactivity
View Solutionएक तत्व 'X' आवर्त सरणी के वर्ग 14 तथा आवर्त 2 से सम्बंधित है, बताइए- (i) परमाणु 'X' में संयोजक इलेक्ट्रॉन की संख्या , (ii ) 'X' की संयोजकता, (iii ) धातु या अधातु (iv ) तत्व का नाम।
View SolutionThe number of valence shell electrons of the most electronegative element is _________.
View SolutionName
(a) three elements that have a single electron in their outermost shells.
(b) two elements that have two electrons in their outermost shells.
(c) three elements with filled outermost shells.
View SolutionIn the following table, the positions of six elements A, B, C, D, E and F are given as they are in the Modern Periodic Table:

On the basis of the above table, answer the following questions:
(i) Name the element which forms only covalent compounds. (ii) Name the element which is a metal with valency three. (iii) Name the element which is a non-metal with valency three. (iv) Out of B and C, whose atomic radius is bigger and why? (v) Write the common name for the family to which the elements D and F belong.
View SolutionGive one example of each :
(a) Metal having valency 2.
(b) Non-metal having valency 2.
(c) Element with three shells, having 4 electrons in the outermost shell.
View Solution
