Draw the resonance structures for the following compounds. Show the electron shift using curved-arrow notation.
(a) C6H5OH (b) C6H5NO2 (c ) CH3CH=CHCHO (d) C6H5−CHO (e ) C6H5−+CH2 (f ) CH3CH=CH+CH2
(a) Resonance structures of C6H5OH:
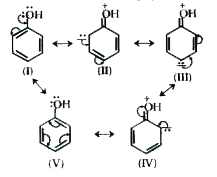
(b) Resonance structures of nitrobenzene:
(c ) Resonance structures of CH3CH=CHCHO
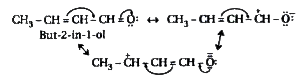
(d) Resonance structures of C6H5−CHO (Benzaldehyde)
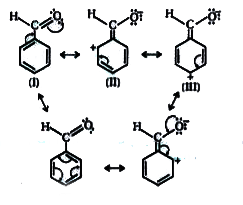
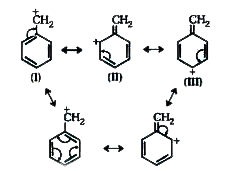
(e ) Resonance structures of C6H5+CH2 (Benzyl Carbocation):
(f) Resonance structure of carbocations of
CH3−CH=CH−+CH2 (But-2-en-1-yl)
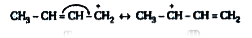
Positive charge is displace by these structure
Topper's Solved these Questions
- ORGANIC CHEMISTRY SOME BASIC PRINCIPLES AND TECHNIQUESBook:KUMAR PRAKASHANChapter:ORGANIC CHEMISTRY SOME BASIC PRINCIPLES AND TECHNIQUESExercise:Section A -Try Your Self-3
Explore 20 Videos
- ORGANIC CHEMISTRY SOME BASIC PRINCIPLES AND TECHNIQUESBook:KUMAR PRAKASHANChapter:ORGANIC CHEMISTRY SOME BASIC PRINCIPLES AND TECHNIQUESExercise:Section-B (Short Questions)
Explore 282 Videos
- ORGANIC CHEMISTRY SOME BASIC PRINCIPLES AND TECHNIQUESBook:KUMAR PRAKASHANChapter:ORGANIC CHEMISTRY SOME BASIC PRINCIPLES AND TECHNIQUESExercise:Section -A(Quantitative Analysis)
Explore 18 Videos
- HYDROGENBook:KUMAR PRAKASHANChapter:HYDROGENExercise:Section-D NCERT Exemplar (Long Answer)
Explore 8 Videos
- QUESTION ASKED IN JEE -2018Book:KUMAR PRAKASHANChapter:QUESTION ASKED IN JEE -2018Exercise:MCQ
Explore 10 Videos
Similar Questions
38cm2N2 gas at 300K temperature and 96pa pressure. In 0.25g organic compound calculate the % of nitrogen. (1 atm .- 101.3 pa)
View SolutionWhat is catenation?
View SolutionExplain the principle and estimation method for carbon and hydrogen in organic compound
View SolutionAtomic hydrogen combines with almost all elements but molecular hydrogen does not. Explain.
View SolutionAccording to molecular orbital theory, which of the following will not be a viable molecule?
View SolutionThe increasing order of reactivity of the following isomeric halides with AgNO3(H2O+alcohol) is
(I) C6H5−CH=CH−CH2−CH2−CI
(II) C6H5−CH=CH−C∣ClH−CH3
(III) C6H5−C∣CI=CH−CH2−CH3
(IV) C6H5−C6H5∣C∣Cl−CH=CH2
View SolutionDraw the resonance structures for the following compounds. Show the electron shift using curved-arrow notation.
(a) C6H5OH (b) C6H5NO2 (c) CH3CH=CHCHO (d) C6H5–CHO (e) C6H5−+CH2 (f) CH3CH=CH+CH2
View SolutionC_(6)H_(5)-CH=CHCHO overset(x)to C_(6)H_(5)CH=CHCH_(2)OH . In the above sequence X can be
View SolutionWrite the name of phenyl-substituded groups:
a. C6H5∣CHme, b C6H5CH2−
C. C6H5CH=CH−, d. C6H5==CHCH2−
View SolutionDraw the resonance structures for the following compounds. Show the electron shift using curved-arrow notation. (a) C_(6)H_(5)OH (b) C_(6)H_(5)NO_(2) (c) CH_(3)CH = CHCHO (d) C_(6)H_(5) - CHO (e) C_(6)H_(5)-overset(+)(C)H_(2) (f) CH_(3)CH = CHoverset(+)(C)H_(2)
View Solutionनिम्नलिखित यौगिकों की अनुनाद-संरचना लिखिए तथा इलेक्ट्रॉनों का विस्थापन मुड़े तीरों की सहायता से दर्शाइए :
(क) C6H5OH
(ख)C6H5NO2
(ग)CH3CH=CHCHO
(घ) C6H5−CHO
(ड़) C6H5−CH2
(च)CH3CH=CH+CH2
View SolutionWrite the intermediate steps for the following reaction. C_(6)H_(5)CH(OH)C-=CH overset(H_(3)O^(+))to C_(6)H_(5)CH=CHCHO
View SolutionDraw resonance strutures of following compounds.
(1) C6H5OH
(2) C6H5NO2
(3) CH3CH=CHCHO
(4) C6H5CHO
(5) C6H5⊕CH2
(6) CH3CH=CH⊕CH2
View SolutionDraw the resonante structures for the folloiwng compounds. Show the elrcton shift using curvedarrow notation :
(1) C6H5OH
(2) C6H5NO2
(3) CH3CH=CHCHO
(4) C6H5−⊕CH2
(6) CH3CH=CH⊕CH2
View SolutionThe reaction
C6H5CHO+CH3CHOdil.NaOH−−−−−→
C6H5CH = CHCHO is called
View Solution

