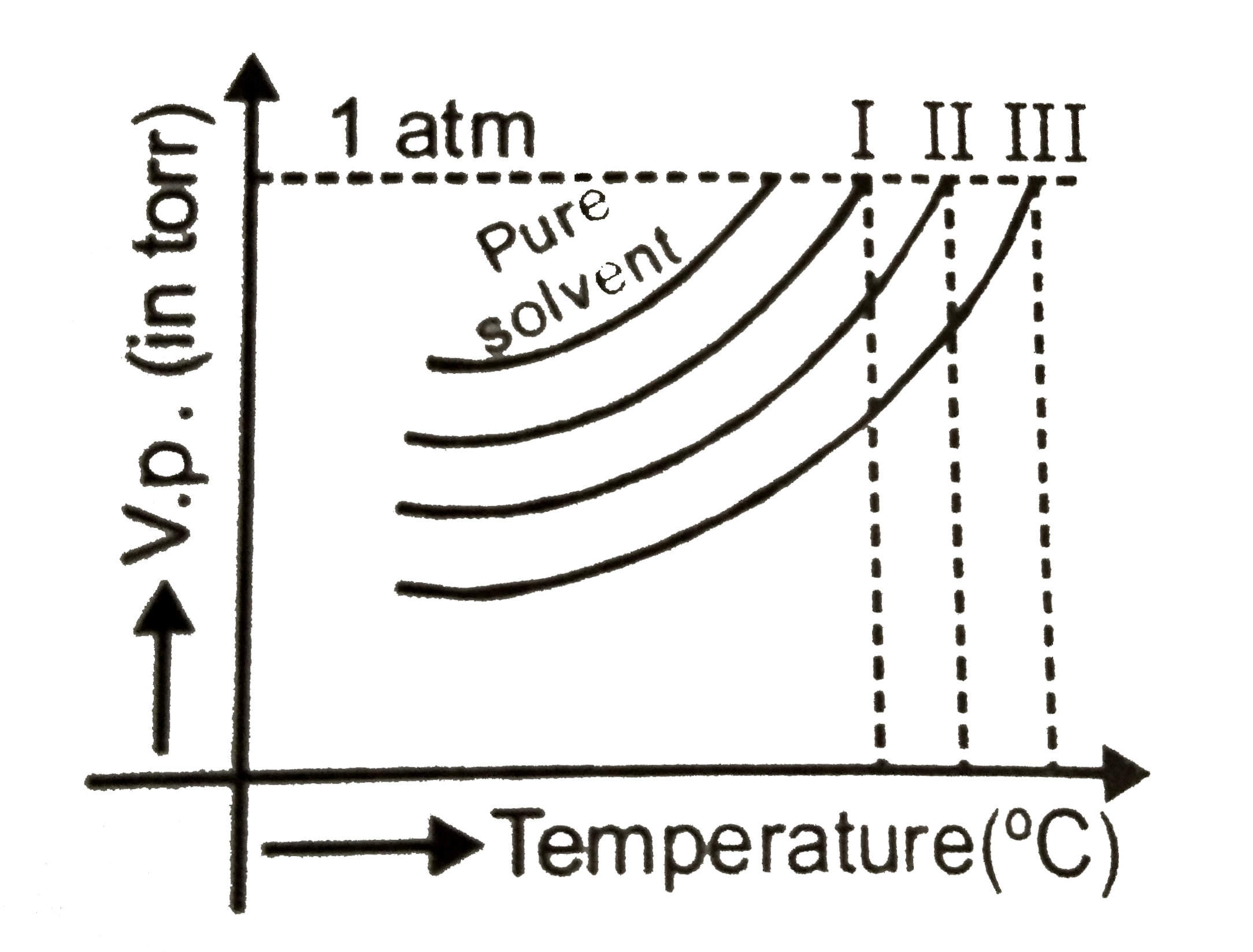
Equimolar solutions in the same solvent have-
A
same boiling points but different freezing points
B
same freezing points but different boiling points
C
same boiling points and same freezing points
D
different boiling points and different freezing points
Video Solution
Text Solution
The correct Answer is:C
Both ΔTf and ΔTb are colligative properties.
|
Similar Questions
Knowledge Check
Equimolar solutions is the same solvents have :
Asame boiling point but different freezing pointBsame freezing point but different boiling pointCsame boiling point and same freezing points .Ddifferent boiling point and freezing points .Equimolar solutions of the same solute in the same solvent have :
ASame boiling point but different freezing pointBSame freezing point but different boiling pointCSame boiling and same freezing pointsDDifferent boiling and different freezing pointsAn open beaker containing a pure solvent and a seconf beaker containing a solution in the same solvent with a non-volatile solute are sealed in a container. Over the period of time
Athe volume of solution increases and the volume of solvent decreasesBthe volume of both solution and solvent increasesCthe volume of solution decreases while volume of solvent increasesDthe volume of both solution and solvent decreases