Explain the significance of three quantum numbers in predicting the position of an electron in an electron in an atom . Solution in Telugu
Each electron quantum number (n ) : The prinicipal quantum number is used to described by a set of three quantum numbers n. L and m1 .
1. Prinicipal quantum number (n ) : The principal quantum number is used to describe th size and energy of the main shell . It is denoted by 'n ' .'n' has positive integer values of 1,2,3,.......... It is used to know the number of orbitals (n2) and electrons in an orbit . (2n2) .
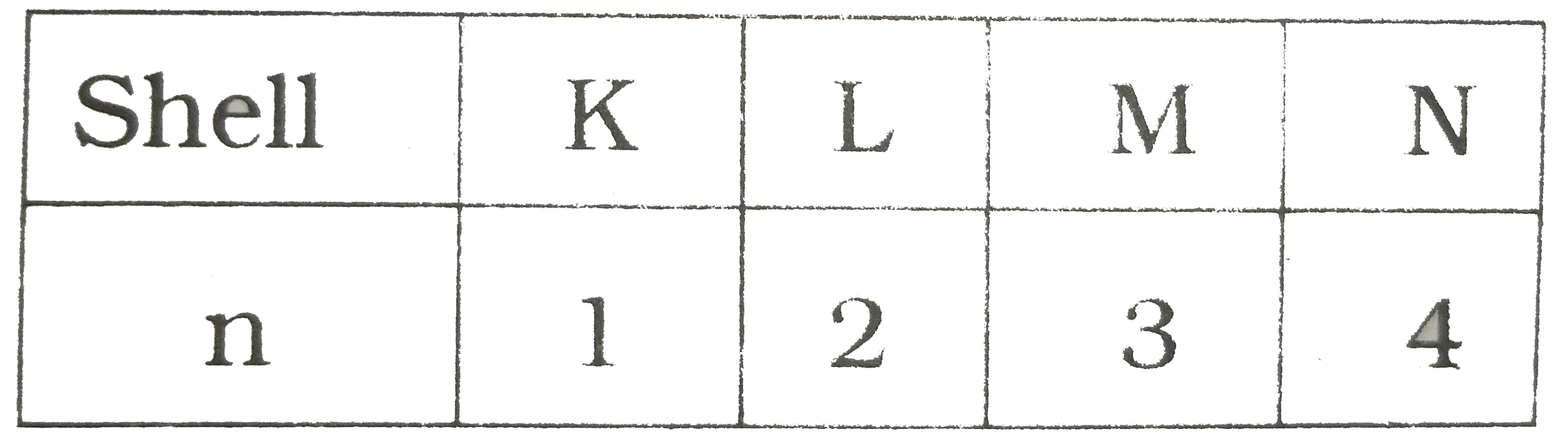
As . 'n' increases the shells becomes larger and the electrons in shells are farther from the nucleus and their energys increaes .
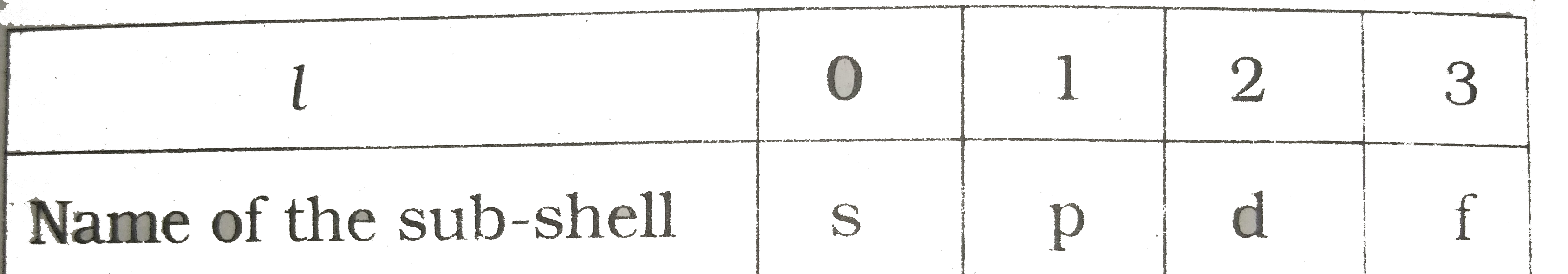
2. The angular - momentum quantum number (l ) : 'l' has integer values from '0' to n-1 for each value of 'n' Each 'l' value reqresents one sub- shell . It is used to describe the shape of an orbit
3. The magnetic quantum number (m1) : The magnetic quantum number (m1) has integer values between -l and +l including zero .
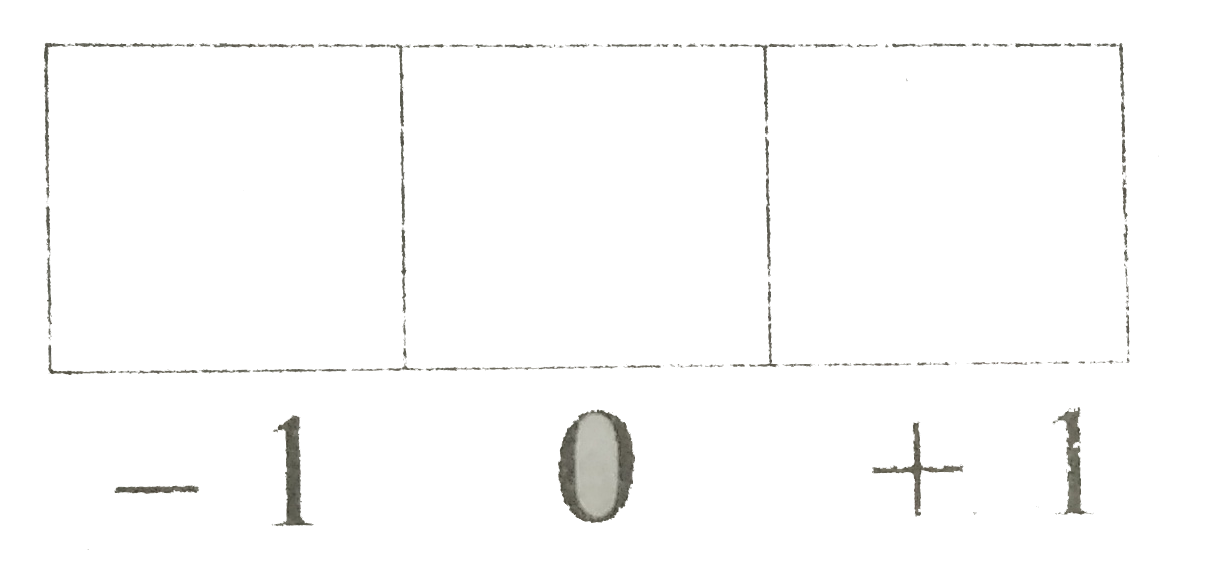
If l=0 the possible mt value is 1.
l= 1, the possible ml value is −1,0and1.
thus for a certain value of l . there are (2l+1) integer values of mf orbitals with different orientations along x,y,z axes labelled as Px.PyandPX orbitals .
predicting the position the position of an elecrton in an atom : If the values of n ,l and ml 2,-1,1 respectively the electron is present in 2px orbital in L- shell .
Similar Questions
Explain the various quantum numbers which completely specify the electron of an atom
View SolutionExplain the various quantum numbers which completely specify the electron of an atom
View SolutionWhat is the significance of spin quantum number in electronic structure of an atom.
View SolutionThe argument of two students is as given : Student 1 : "We need four quantum numbers to represent an electron in a multi- electron atom." Student 2 : " We ned only first three quantum numbers to represent an electron in a multi- electron atom." a) Which are the four quantum numbers?
View SolutionThe argument of two students is as given : Student 1 : "We need four quantum numbers to represent an electron in a multi- electron atom." Student 2 : " We ned only first three quantum numbers to represent an electron in a multi- electron atom." c) Write the possible four quantum numbers of the valence electron of Na atom.
View SolutionQuantum numbers are a set of four numbers used to designate electron in an atom. ii) Give the quantum numbers of the valence electron of an atom with atomic number 13.
View SolutionQuantum numbers are the address of an electron in an atom. Justify the statement by explaining different quantum numbers.
View SolutionQuantum number gives address of electrons. Explain the quantum number which determines:
i) Distance of electron from nucleus.
View Solution
