Explain the terms inductive and Electromeric effects. Which electron displacement effect explains the following correct orders of acidity of the carboxylic acids?
(a) Cl3CCOOH>Cl2CHCOOH>ClCH2COOH (b) CH3CH2COOH>(CH3)2CHCOOH>(CH3)3C.COOH
(a) Cl3COOH>Cl2CHCOOH>ClCH2COOH: These correct order of acidity is explain by negative inductive effect (-I) of Cl.
Cl group containing electron attracting inductive effect
As the no. of Cl group is more the electron attracting induction is more.
These inductive effect displace in C-C, C-O and O-H bond. So electron of O-H bond remain near to O and away from H and H and O−H bond become Oδ−−Hδ+ polar. The disability of removed of H+ is maximum in Cl3CCOOH
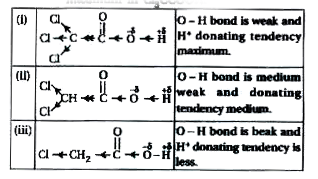
(i)→(ii)→(iii), as the no. of Cl decreases, the tendency of removal of O-H bond decreases, so acidic strength is also decreases.
(b) CH3CH2COOH>(CH3)2CHCOOH>(CH3)3CCOOH: In these the alkyle groups are containing electron donating (repulsive) (+I) inductive effect. As the number of alkyl group −CH3 is increase, then the electron repulsion is also increases. And therefore electron are comes near to O-H bond and O-H bond becomes weak. H+ donating tendency is decrease so, acidic strength also decrease . THe (+I) effect of -CH3 displace in bond is as under.
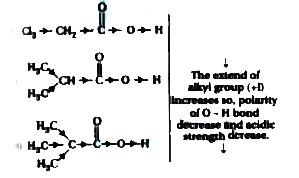
Similar Questions
Explain the terms Inductive and Electromeric effects. Which electron displacement effect explains the following correct orders of acidity of the carboxylic acids?
(a) Cl3CCOOH>Cl2CHCOOH>ClCH2COOH
(b) CH3CH2COOH>(CH3)2CHCOOH>(CH3)3C.COOH
View SolutionExplain the terms Inductive and Electromeric effects. Which electron displacement effect explains the following correct orders of acidity of the carboxylic acids?
(a) Cl3CCOOH>Cl2CHCOOH>ClCH2COOH
(b) CH3CH2COOH>(CH3)2CHCOOH>(CH3)3C.COOH
View Solution
Knowledge Check
Which of the following is the correct orderr of acidity of carboxylic acids?
(i) Cl3CCOOH>Cl2CHCOOH>ClCH2COOH
(ii) CH3CH2COOH>(CH3)2CHCOOH>(CH3)3CCOOH
(iii) F2CHCOOH>FCH2COOH>ClCH2COOHA(i) and (ii)B(ii) and (iii)C(i) and (iii)D(i), (ii) and (iii)Which of the following is the correct orderr of acidity of carboxylic acids?
(i) Cl3CCOOH>Cl2CHCOOH>ClCH2COOH
(ii) CH3CH2COOH>(CH3)2CHCOOH>(CH3)3CCOOH
(iii) F2CHCOOH>FCH2COOH>ClCH2COOHA(i) and (ii)B(ii) and (iii)C(i) and (iii)D(i), (ii) and (iii)Which one of the following is the correct order of acidic strength?
(i) Cl3C−COOH
(ii) Cl2CH−COOH
(iii) ClCH2−COOH
(iv) CH3−COOHAiv>iii>ii>iBi>ii>iii>ivCi>iii>ii>ivDiv>ii>iii>i
Which one of the following is the correct order of acidic strength?
(i) Cl3C−COOH
(ii) Cl2CH−COOH
(iii) ClCH2−COOH
(iv) CH3−COOH
View SolutionArrange the following as stated :
'Increasing order of acidic strength'.
ClCH2COOH,CH3CH2COOH,ClCH2CH2COOH,(CH3)2CHCOOH,CH3COOH.
View SolutionArrange the following carboxylic acids in the decreasing order of reactivites
(1) CH3COOH (2) ClCH2COOH
(3) Cl2CHCOOH (4) Cl3CCOOH
View SolutionPlace the following in the correct order of acidity
1. CH≡ C COOH 2. CH2≡CHCOOH
3. CH3CH2COOH
View SolutionThe acid strength of the following carboxylic acids increases in the order ___________.
HCOOH>CH3COOH,CH3CH2COOH,CH3CH2CH2COOH
View Solution

