Explain with suitable example that the resonance structure of molecule do not represent real structure and they are hypothetical
Text Solution
There are many organic molecules whose behaviour cannot be explained by a single Lewis structure. An example is that of benzene. Its cyclic structure containing alternating C-C single and C=C double bonds shown is inadequate for explaining its characteristic properties. As per the above representation, benzene should exhibit two different bond lengths, due to C-C single and C=C double bonds. Experimentally by X-ray diffraction, in benzene all C-C bond length is 139pm

However, as determined experimentally benzene has a uniform C-C bond distances of 139 pm, a value intermediate between the C-C single (154pm) and C=C double (134pm) bonds. Thus, the structure of benzene cannot be represented adequately by the above structure. Benzene can be represented equally well by the energetically identical structures (I) and (II).
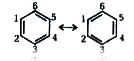
Therefore, according to the resonance theory the actual structure of benzene cannot be adequately represented by any of these structures, rather it is a hybrid of the two structures (I and II) called resonance structures as figure (III).

The resonance structures (canonical structures or contributing structures) are hypothetical and individually do not represent any real molecule. They contribute to the actual structure in proportion to their stability.
|
Similar Questions
किसी अणु की संरचना को दर्शाने के लिए आवश्यक किसी अणु के केनोनिकल या अनुनादी संरचनाएँ निम्न में से किस नियम का पालन करती हैं?
View SolutionGive resonance structure of nitromethane and explain its real structure
View SolutionWhat is Resonance structure ? Why the resonances structure required explain with' suitable example. OR Give resonance of O_(3) , its requirement, delimitation and advantages
View SolutionExplain with suitable example that the resonance structure of molecule do not represent real structure and they are hypothetical
View SolutionGive resonance structure of nitromethane and explain its real structure
View SolutionSelect the structures that are not resonance structures of the same molecule .
View SolutionExplain the resonance structure of benzene. What do you understand by resonance hybrid?
View Solution

