[Fe(H2O)6]3+ is strongly paramagnetic whereas [Fe(CN)6]3− is weakly paramagnetic Explain.Solution in Telugu
Video Solution
Text Solution
Outer configuration of 26Fe=3d64s2. In both the complex Fe is present as Fe3+ ion.
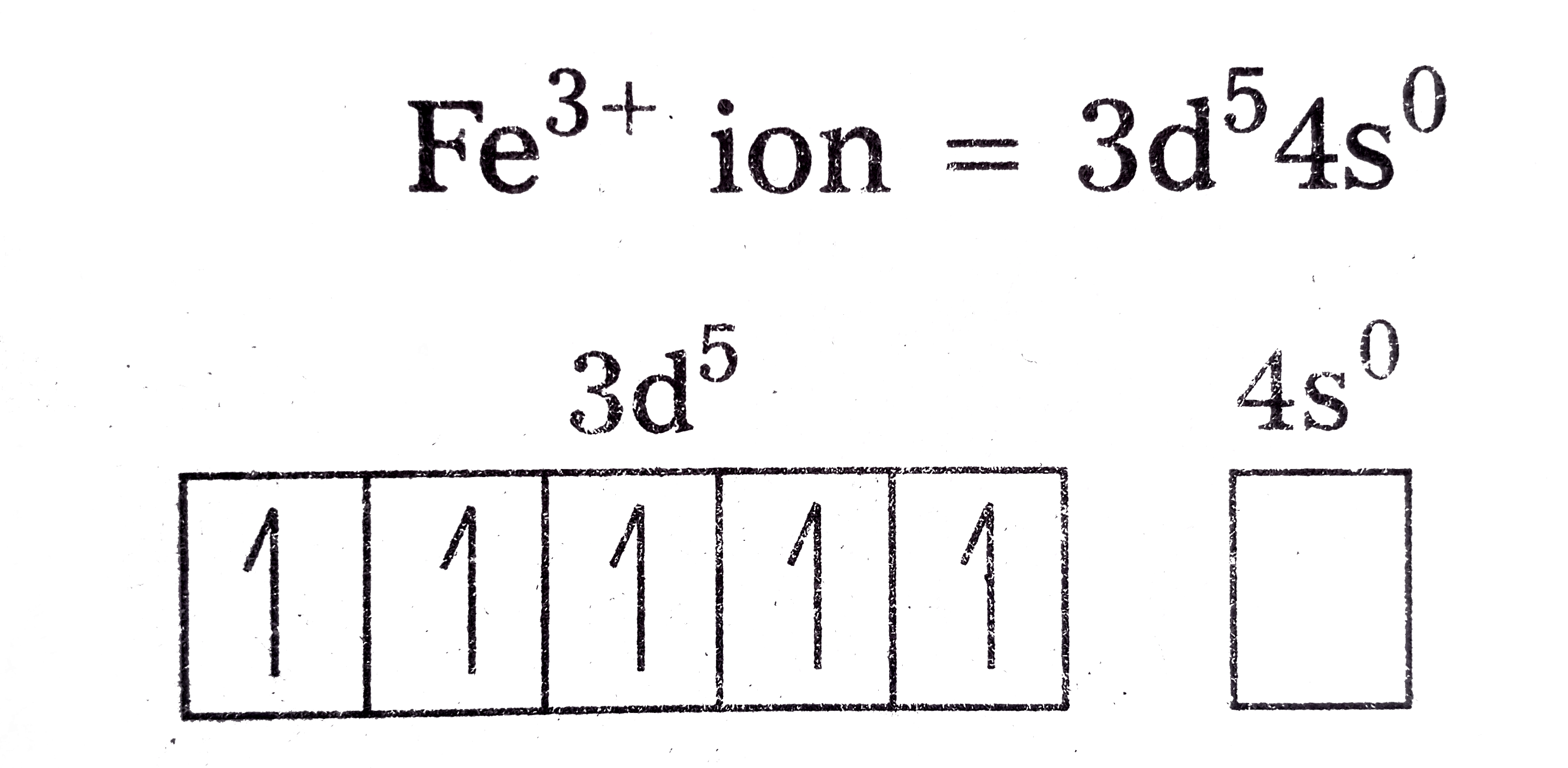
In the presence of CN− (a strong field ligand) the 3d-electrons pair up leaving only one unpaired electron. The hybridization is d2sp3 forming an inner orbital complex.
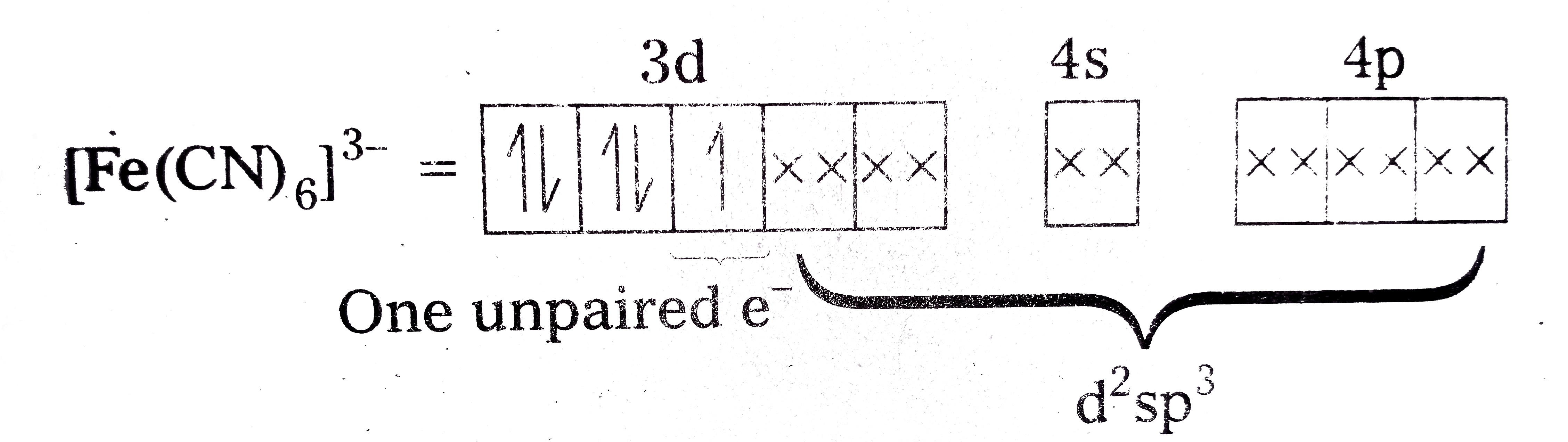
In the presence of H2O, (a weak field ligand), 3d-electrons do not pair up. The hybridization is sp3d2 forming an outer orbital complex containing five unpaired electrons. Hence, it is strongly paramagnetic.

|
Similar Questions
[Fe(H2O)6]3+ प्रबल अनुचुंबकिय है जबकि [Fe(CN)6]3− दुर्बल अनुचुंबकिय। समझाइये।
View Solution[Fe(H2O)6]3+ is strongly paramagnetic whereas [Fe(CN)6]3− is weakly paramagnetic . Explain.
View Solution[Fe(H2O6]3+ is strongly paramagnetic whereas [Fe(CN)6]3− is weakly paramagnetic. Explain.
View Solution[Fe(H_(2)O)_(6)]^(3+) is strongly paramagnetic whereas [Fe(CN)_(6)]^(3-) is weakly paramagnetic. Explain.
View SolutionExplain the following :
[Fe(H2O)6]3+ is more paramagnetic than [Fe(CN)6]3−.
View Solution[Fe(H_2O)_6]^(3+) is strongly paramagnetic whereas [Fe(CN)_6]^(3-) is weakly paramagnetic. Explain.
View Solution[Fe(H2O)6]3+ is strongly paramagnetic whereas [Fe(CN)6]3− is weakly paramagnetic. Explain.
View Solution[Fe(H_2O)_6]^(3+) is strongly paramagnetic whereas {Fe(CN)_6]^(3-) is weakly paramagnetic Expain?
View Solution
