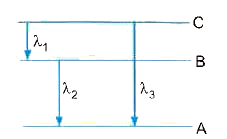
For a certain atom, there are energy levels A, B, C corresponds to energy values EA<EB<EC . Choose the correct option if λ1,λ2,λ3 are the wavelength of radiations corresponding to the transition from C to B, B to A and C to A respectively.
λ3=λ1+λ2
λ3=λ1λ2λ1+λ2
λ1+λ2+λ3=0
3λ2=λ3+2λ2
The correct Answer is:B
The energy difference between levels C and A will equal to the sum of energy difference between levels C & B levels B&A.
EB−EA=hcλ1
EC−EB=hcλ2
EC−EA=hcλ3
or1λ3=1λ1+1λ2
λ3=λ1λ2λ1+λ2
Topper's Solved these Questions
Similar Questions
The third viral coefficient for real gas is 2×10−2(L mole−1). The molar volume of gas at 27∘C and 5 atm Pressure may be-
View SolutionThe coagulating power of electrolytes having ions Na2+>Al3+andBa2+ for arsenic sulphide sol increase in the order,
View SolutionEnergy levels A,B,C of a certain atom corresponding to increasing values of energy i.e., EA<EB<EC. If λ1,λ2,λ3 are the wavelengths of radiations corresponding to the transitions C to B,B to A and C to A respectively, which of the following options is correct?
View SolutionEnergy levels A. B, C. of a certain atom correspoinds to increasing value fo enrgy i.e., E_A lt E_B lt E_C If lambda _1, lambda_2 , and lambda_3 are the wavelengths of radistions corresponding to the transitions ( C) to B, B to (A) and ( C) to (A) respectively wich fo the following wstatemnt is correct .
View SolutionEnergy levels A,B and C of a certain atom correspond to increasing values of energy i.e. EA<EB<EC .If λ1,λ2,λ3 are the wavelengths of radiation corresponding to transition C to B,B to A and C to A respectively, which of the following statements is correct ?
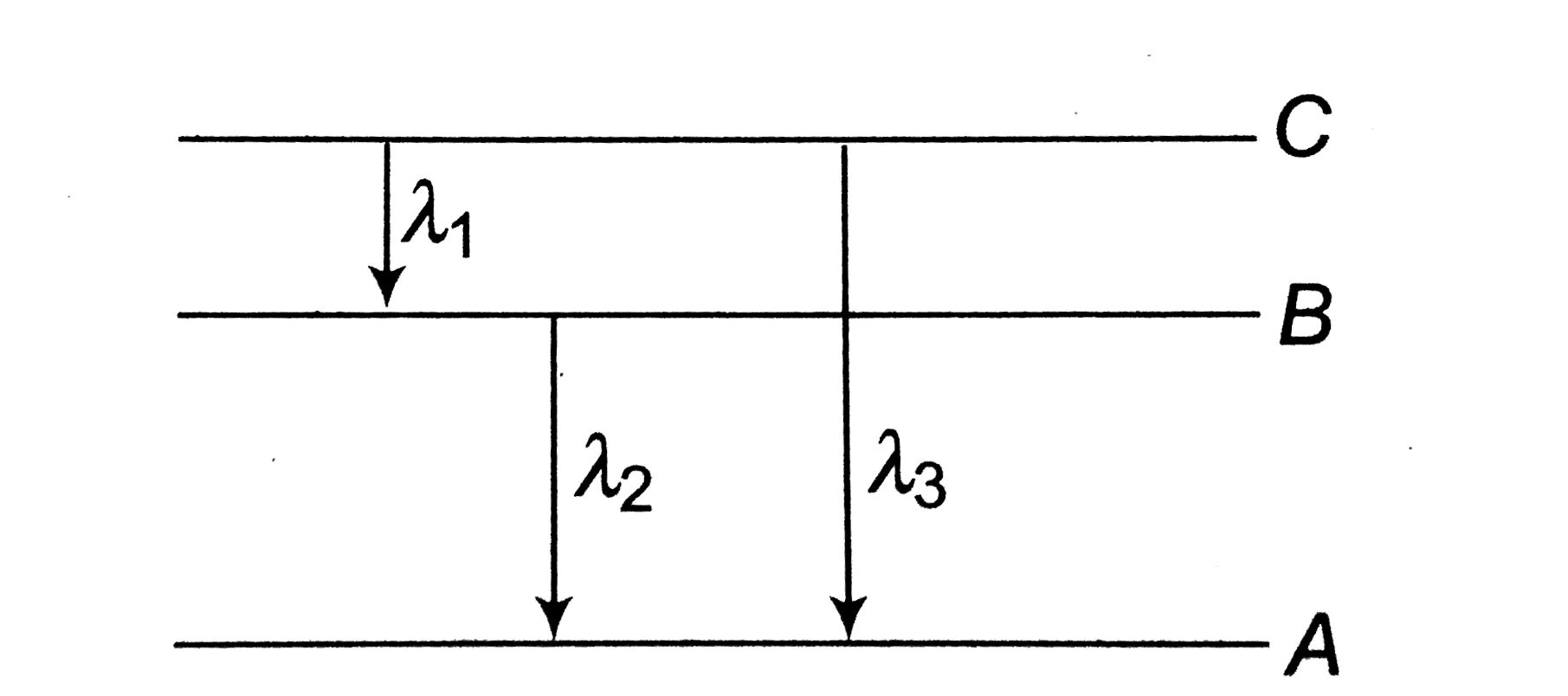
View SolutionEnergy levels A,B,C of a certain atoms corresponding to increasing values of energy level i.e., EA<EB<EC. If λ1,λ2 and λ3 are the wavelengths of radiations corresponding to the transitions C to B,B to A and C to A respectively which of the following statement is correct?
View SolutionEnergy levels A, B and C of a certain atoms correspond to increasing values of energy, i.e. EA<EB<Ec. If λ1,λ2,λ3 are the wavelengths of radiation corresponding to the transitions C→B, B→A and C→A respectively, then :
View SolutionEnergy levels A,B,C of a certain atom correspond to increasing values of energy i.e., EA<EB<EC. If λ1,λ2,andλ3 are the wavelength of radiations corresponding to the transitions C to B , B to A and C to A , respectively. Which of the following statement is correct ?
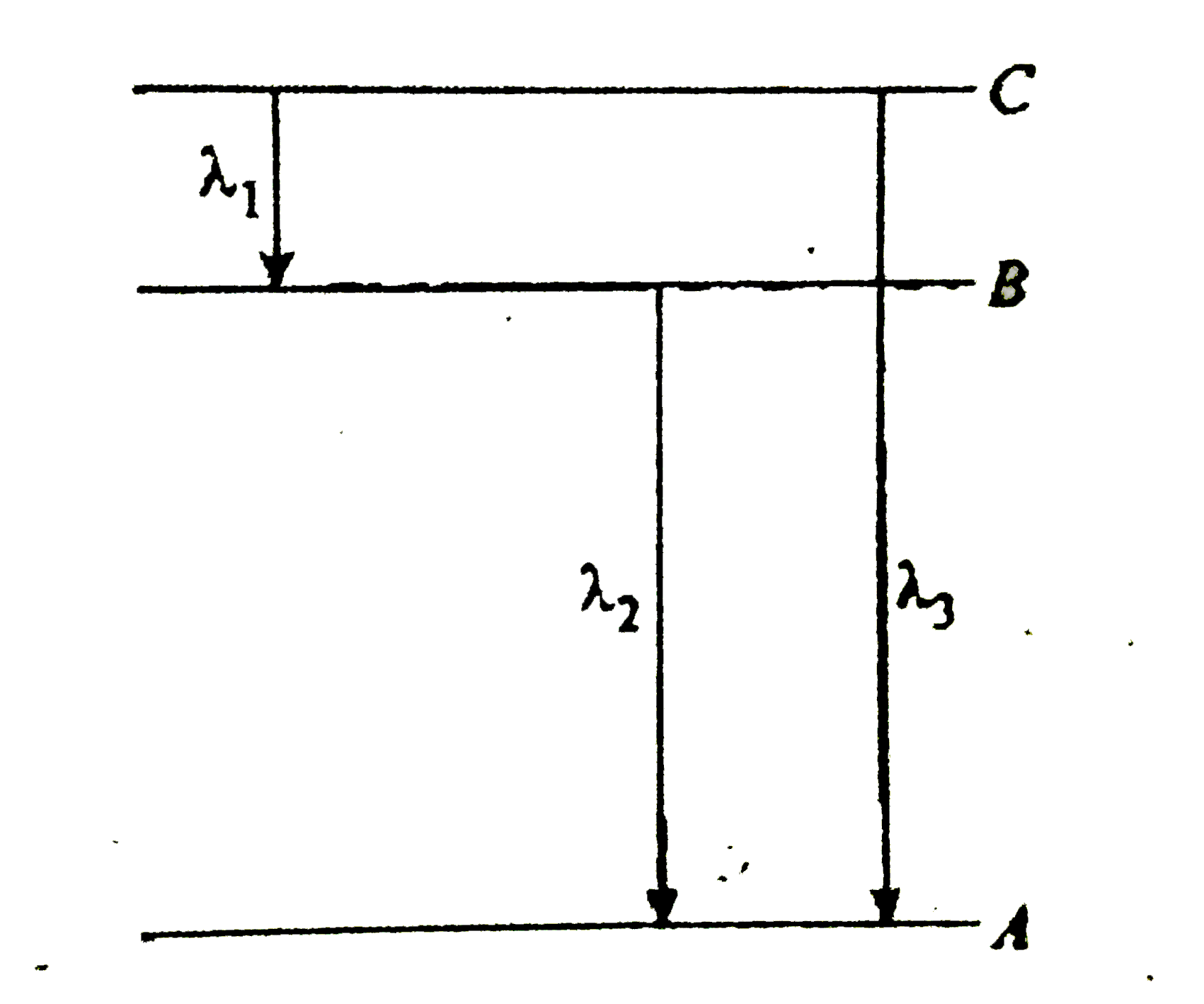
View SolutionThree ascending energy states of an atom are A,B,C. The wavelengths of emitted radiations due to transition from C to B and from B to A are λ1andλ2 respectively. What will be the wavelength of emitted radiation due to transition from C to A ?
View SolutionFor a certain atom, there are energy levels A, B, C corresponds to energy values E_A Choose the correct option if lamda_1,lamda_2,lamda_3 are the wave length of radiations corresponding to the transition from C to B, B to A and C to A respectively. .
View SolutionEnergy levels A,B and C of a certain atom correspond to increasing values of energy i.e. EA<EB<EC .If λ1,λ2,λ3 are the wavelengths of radiation corresponding to transition C to B,B to A and C to A respectively, which of the following statements is correct ?

View SolutionThe energy levels A, B, C of a particular atom with respect to the values of varying energies E_A lt E_B lt E_C Huh. If lambda_1.lambda_2व lambda_3 If the wavelengths of the radiations are relative to the transition from C to B, B to A and C to A respectively, then which statement is true?
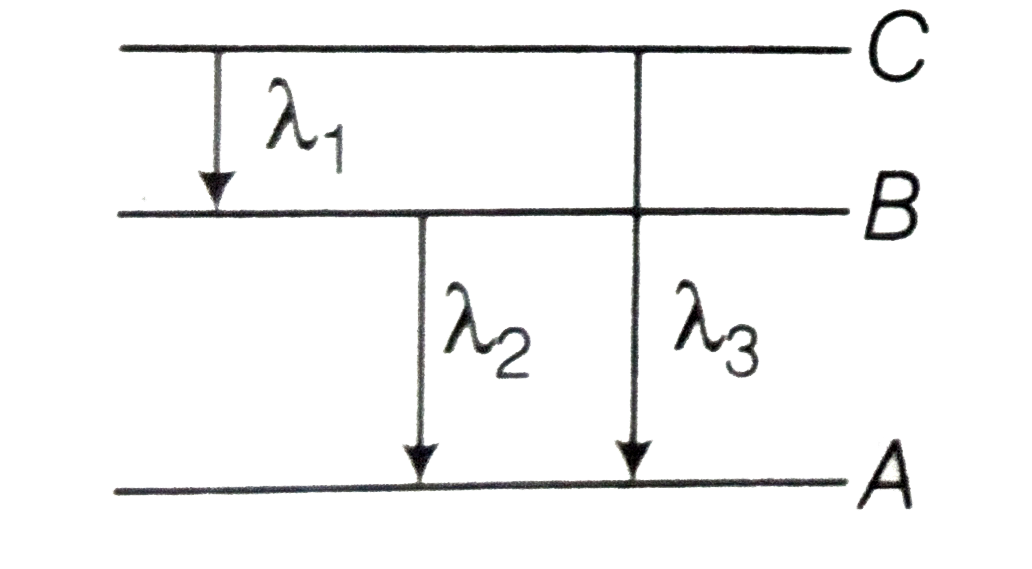
View SolutionEnergy levels A, B, C of a certain atom corresponding to increasing values of energy are EA<EB<ECλ1,λ2,λ3 are the corresponding wavelengths of the radiations corresponding to the transitions C to B, B to A and C to A respectively. Which of the following statements is true ?
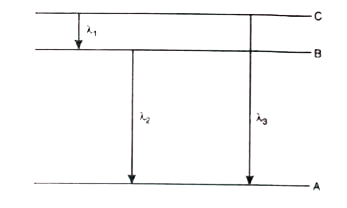
View SolutionEnergy levels A, B and C of a certain atom correspond to increasing values of energy i.e., EA<EB<EC .If λ1,λ2 and λ3 are the wavelengths of radiations corresponding to the transitions Cto B, B to A and C to A respectively as shown in Fig. 12.04, then which of the following statement is correct?
View Solutionएक X-ray ट्यूब 1.24 मिलियन वोल्ट पर संचालित होती है। उत्पादित फोटॉन की सबसे छोटी तरंगदैर्घ्य होगी :
View SolutionEnergy levels A,B,C of a certain atom corresponding to increasing values of energy i.e., EA<EB<EC. If λ1,λ2,λ3 are the wavelengths of radiations correspnding to the transitions C to B,B to A and C to A respectively, which of the following statements is correct?

View Solution

