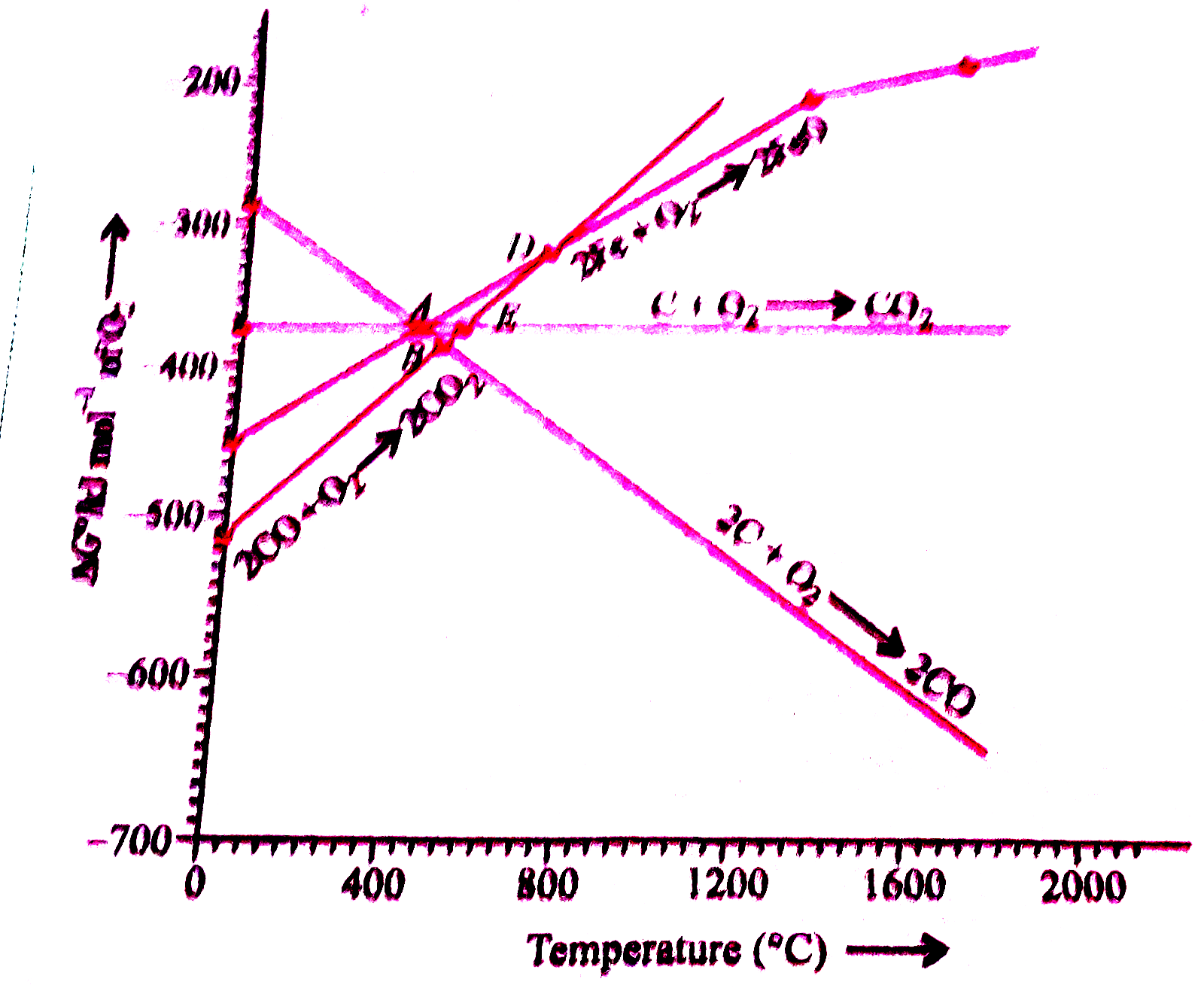
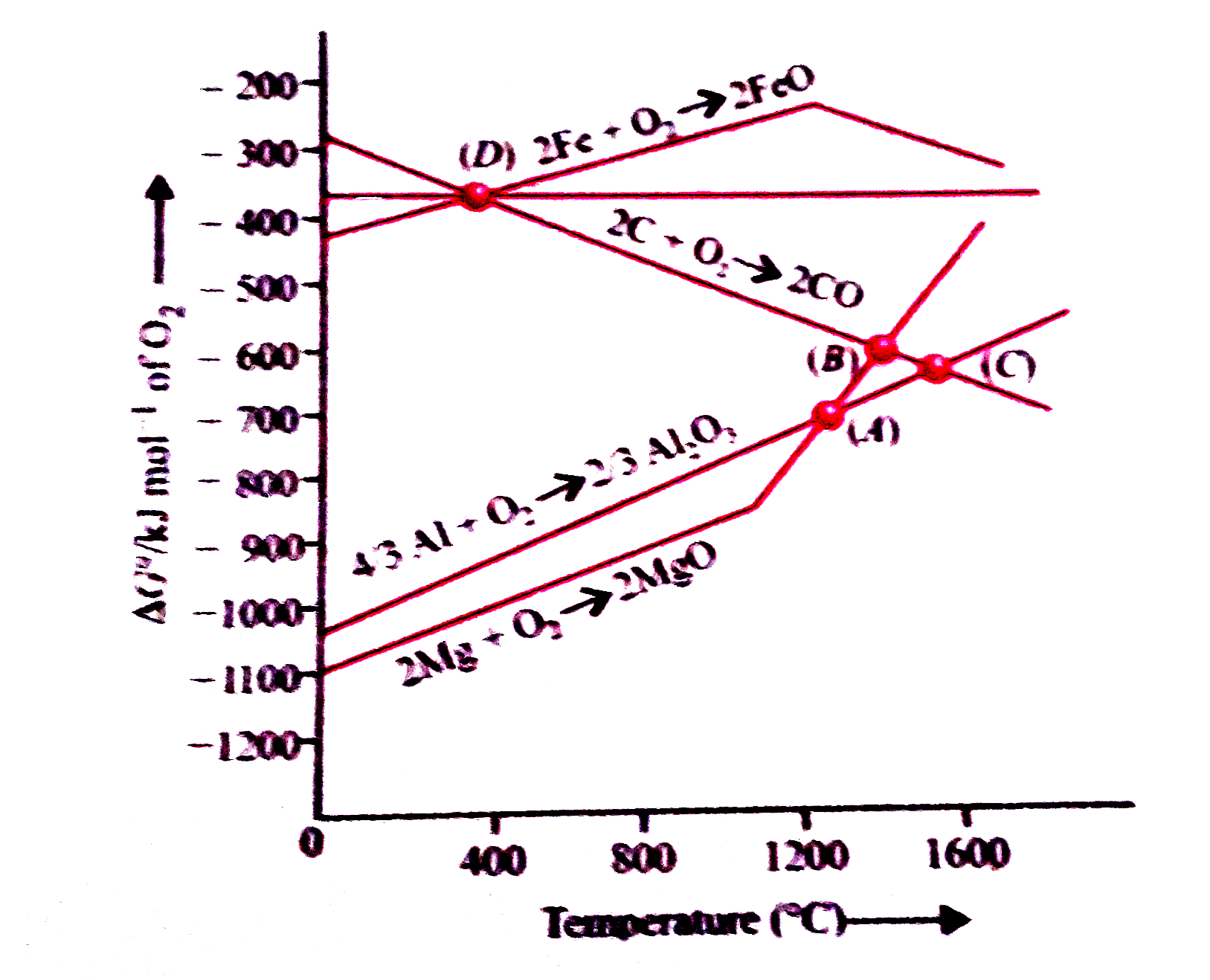
For the reduction of Feo at the temperature corresponding to point D, which of the following statements is correct?
ΔG∘ value for the overall reduction reaction with carbon monoxide is zero.
ΔG∘ value for the overall reduction reaction with a mixture of 1 mol carbon and 1 mol oxygen is positive.
ΔG∘ value for the overall reduction reaction with a mixture of 2 mol carbon and 1 mol oxygen will be positive.
ΔG∘ value for the overall reduction reaction with carbon monoxide is negative.
The correct Answer is:A
At point D,ΔG∘(CO,CO2)=ΔfGΘ(Fe,FeO)
Thus overall value of ΔG∘ will be zero.
Similar Questions
Knowledge Check
For the reaction
A+B→2C+D which of the following statements is/are correct?ARate of disappearance of B = 1/2 x rate of appearance of CBRate of disappearance of B =1/2 x rate of appearance of CCRate of disappearance of A = rate of appearance of BDRate of disapperance of A = rate of disapperance of B.Which one of the following statements about temperature is correct?
ATemperature decreases with height in the stratosphere.BTemperature is constant at different heights in the StratosphereCTemperature increases with height in the troposphere at an average rate of 6.5∘C per kilometer.DTemperature decreases with height in the troposphere eat an average rate of 6.4∘C per kilometreWhich of the following reduction processes are correct
AFe2O3+CReduction−−−−−→FeBZn+CReduction−−−−−→ZnCSnO2+CReduction−−−−−→SnDPbO+CReduction−−−−−→Pb
Which of the following statement is correct for 3d-transition element
View SolutionWhich of the following statements is correct regarding self reduction?
View SolutionWhich of the following statements is correct regarding self reduction?
View SolutionWhich of the following statements is correct regarding self reduction?
View SolutionA liquid mixture ohaving composition corresponding to point Z in the figure shown is subjected to distillation at constant pressure. Which of the following statements is correct about the process?
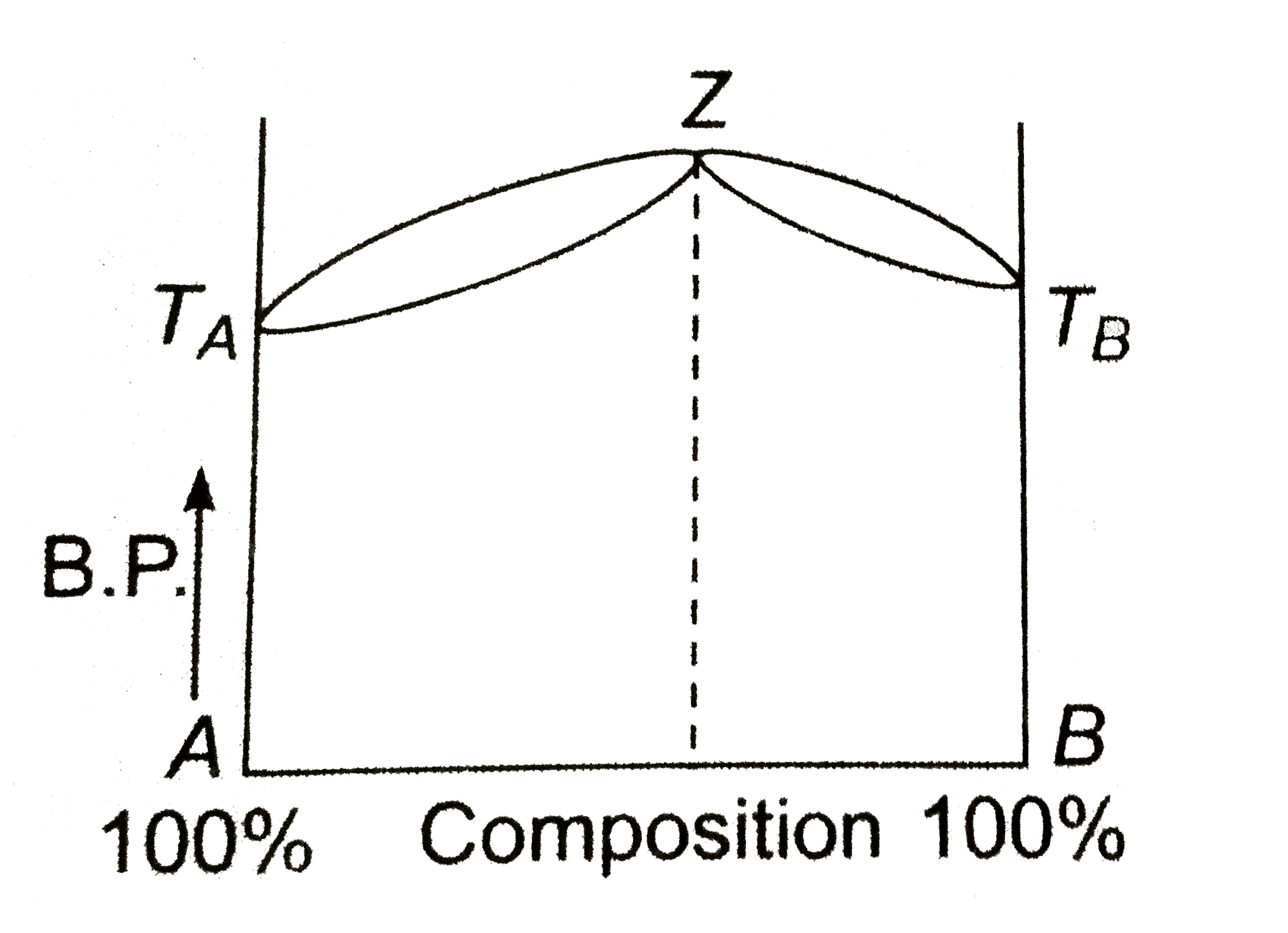
View Solution