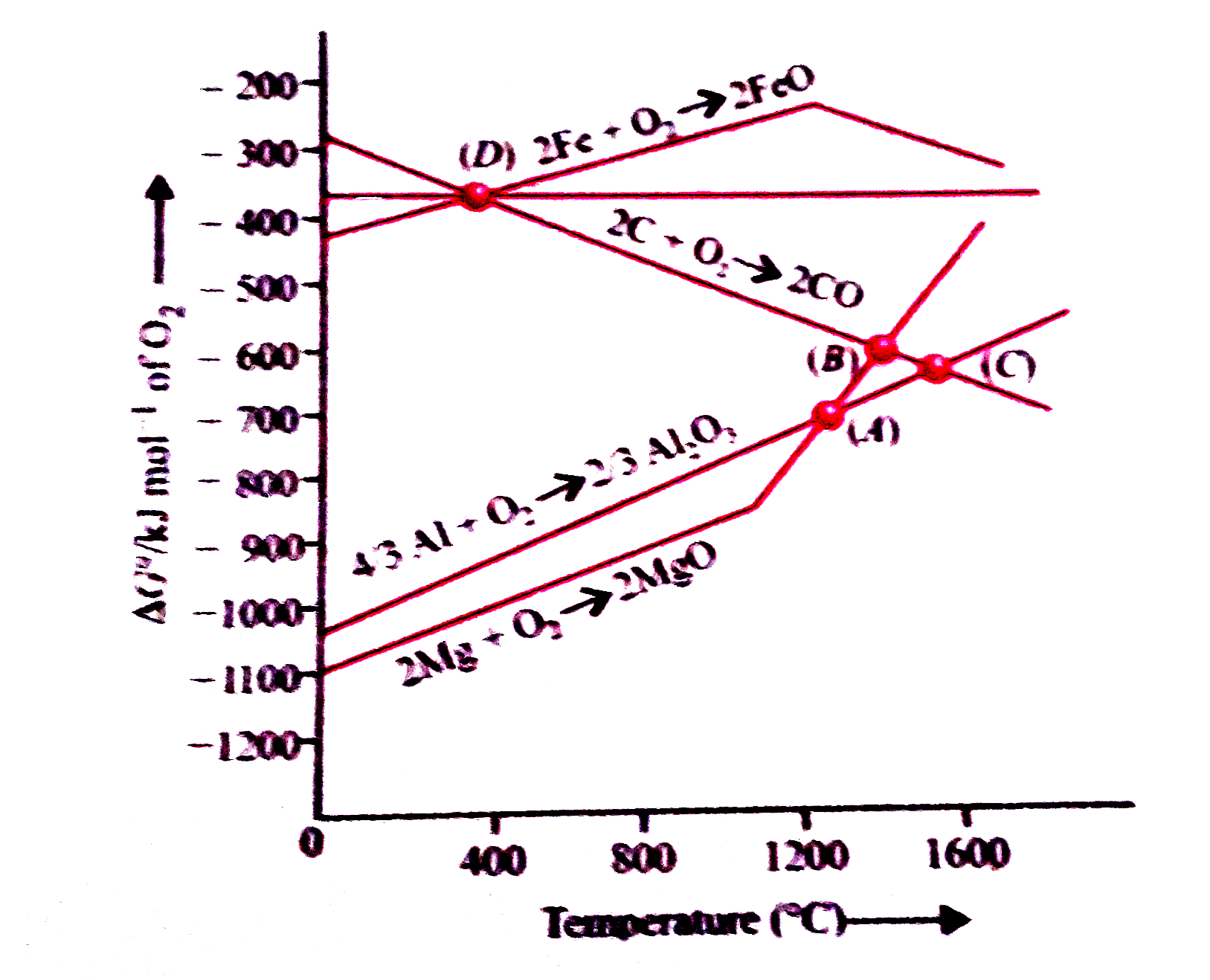
For the reduction of FeO with C at the temperature corresponding to (D) , which of the following statements is correct ?
A
carbon reduces FeO below the temperature at point (D)
B
ΔG∘ value for overall reduction with CO is zero.
C
ΔG∘ value for the reaction at point (D) is positive .
D
Below point (D) , for the metal exists in molten state.
Video Solution
Text Solution
The correct Answer is:B
ΔG∘reactant=ΔG∘product at the point of intersection , so ΔG∘ become zero for the overall reaction.
|
Topper's Solved these Questions
- GENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTSBOOK - NCERT FINGERTPS ENGLISHCHAPTER - GENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTSEXERCISE - HOTS
7 Videos
- GENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTSBOOK - NCERT FINGERTPS ENGLISHCHAPTER - GENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTSEXERCISE - NCERT EXEMPLAR PROBLEMS
13 Videos
- ELECTROCHEMISTRYBOOK - NCERT FINGERTPS ENGLISHCHAPTER - ELECTROCHEMISTRYEXERCISE - Assertion And Reason
15 Videos
- HALOALKANES AND HALOARENESBOOK - NCERT FINGERTPS ENGLISHCHAPTER - HALOALKANES AND HALOARENESEXERCISE - NCERT Exemplar
27 Videos
Similar Questions
Knowledge Check
Which of the following statement is correct ?
AEcell and ΔrG of cell reaction both are extensive properties.BEcell and ΔrG of cell reaction both are intensive properties.CEcell is an intensive property while ΔrG of cell reaction is an extensive property.DEcell is an extensive property while ΔrG of cell reaction is an intensive property.Which of the following statement is correct?
AC2H5Br reacts with C2H5ONa to form diethyl etherBC2H5Br reacts with AgCN to form ethyl cyanideCC2H5Br reacts with alc. KOH to form C2H5OHDC2H5Br on treatment with Na gives C2H5OHWhich of the following statement is correct?
AThe rate of a reaction decreases with passage of time as the concentration of reactants decreases.BThe rate of a reaction is same at any time during the reactionCThe rate of a reaction is independent of temperature changeDThe rate of a reaction decreases with increase in concentration of reactant(s)
Which of the following statement is not correct ?
View SolutionWhich of the following statements is not correct?
View SolutionWhich of the following statements is not correct?
View SolutionWhich of the following statement is correct?
View SolutionWhich of the following statements is not correct ?
View Solution

