From the following flowsheet for the extraction of pure metal, answer the given questions.
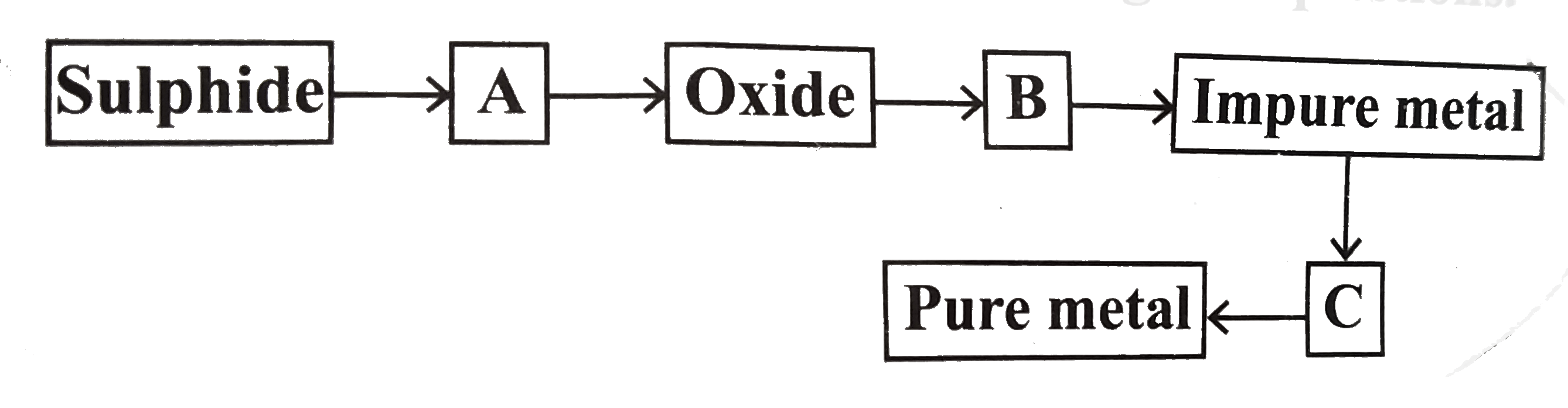
(iv) Some of the following metals are obtained by electrolysis of their fused salts : Al,Na,Cu,Ag,Mg,Ca. These metals are :
Cu,Ag
Al,Na,Cu
Ag,Mg,Ca
Al,Na,Mg,Ca
The correct Answer is:D
Step by step video, text & image solution for From the following flowsheet for the extraction of pure metal, answer the given questions. (iv) Some of the following metals are obtained by electrolysis of their fused salts : Al, Na, Cu, Ag, Mg, Ca. These metals are : by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Similar Questions
Knowledge Check
From the following flowsheet for the extraction of pure metal, answer the given questions.
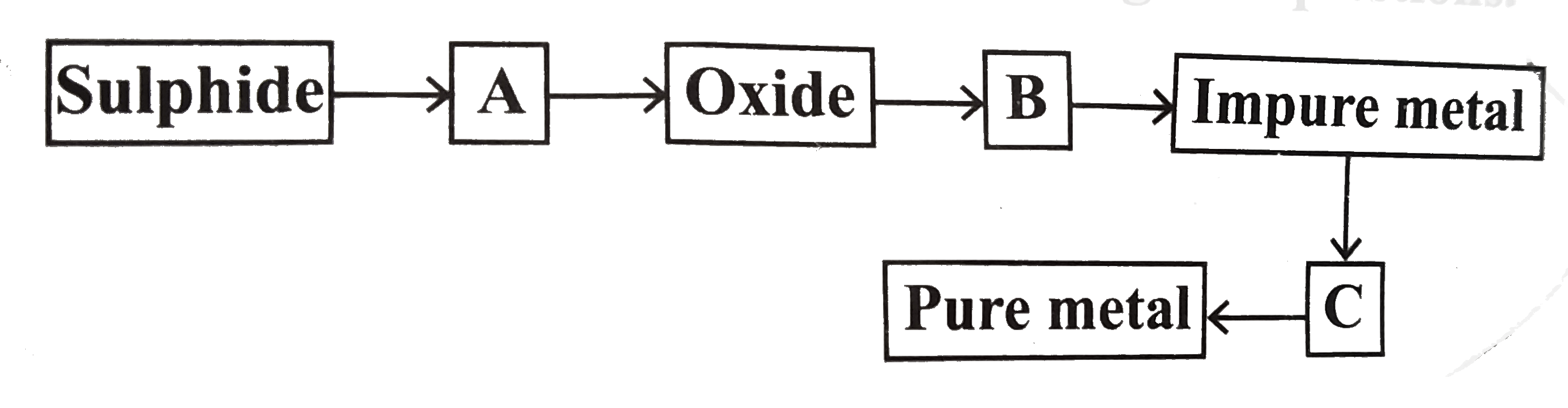
(i) Step A is :ARoastingBSmeltingCCalcinationDBessemerisaionFrom the following flowsheet for the extraction of pure metal, answer the given questions.
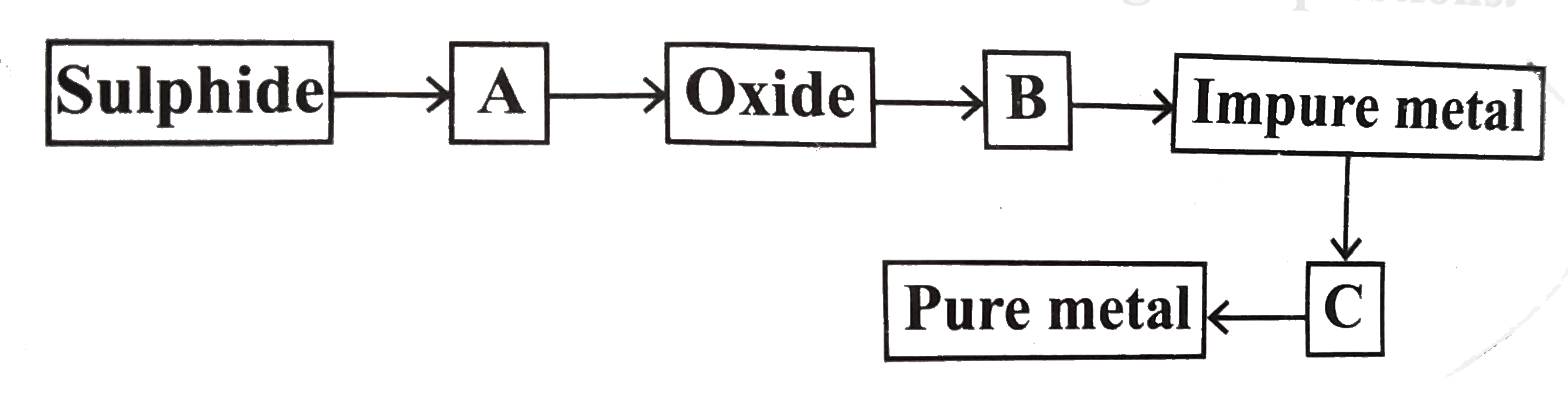
(iii) Impure Cu metal is also obtained :ABy self-reduction during roasting of CuSBBy reduction of CuO with H2CBy reduction of CuO with AlDBy electrolysis of Cu2+ solutionFrom the following flowsheet for the extraction of pure metal, answer the given questions.
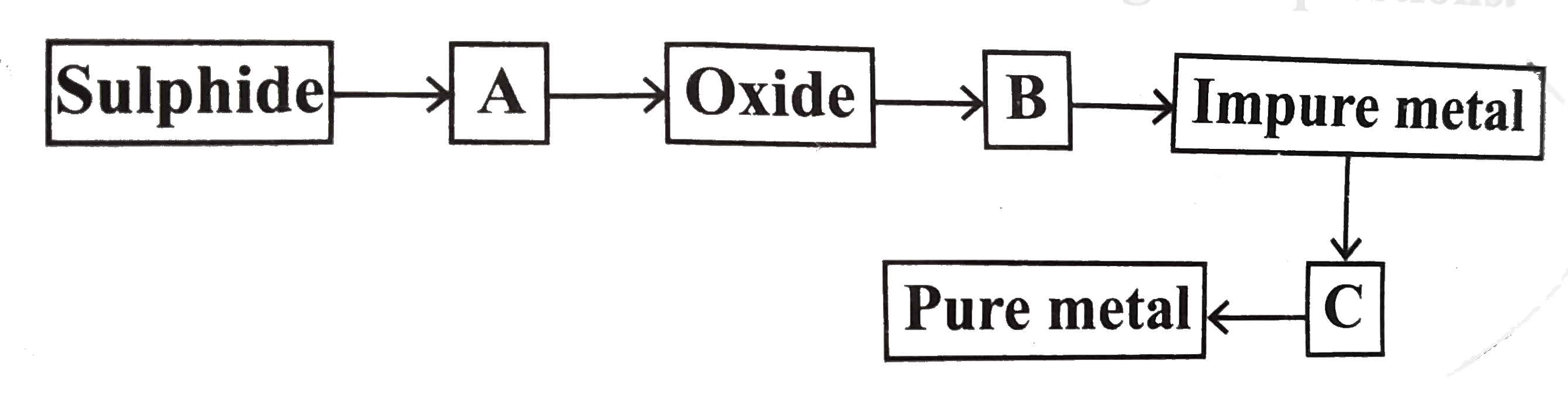
(vi) following reaction is not involved intermite process :A3Mn3O4+8Al→9Mn+4Al2O3BCr2O3+2Al→Al2O3+2CrC2Fe+Al2O3→2Al+Fe2O3DB2O3+2Al→2B+Al2O3
From the following flowsheet for the extraction of pure metal, answer the given questions.
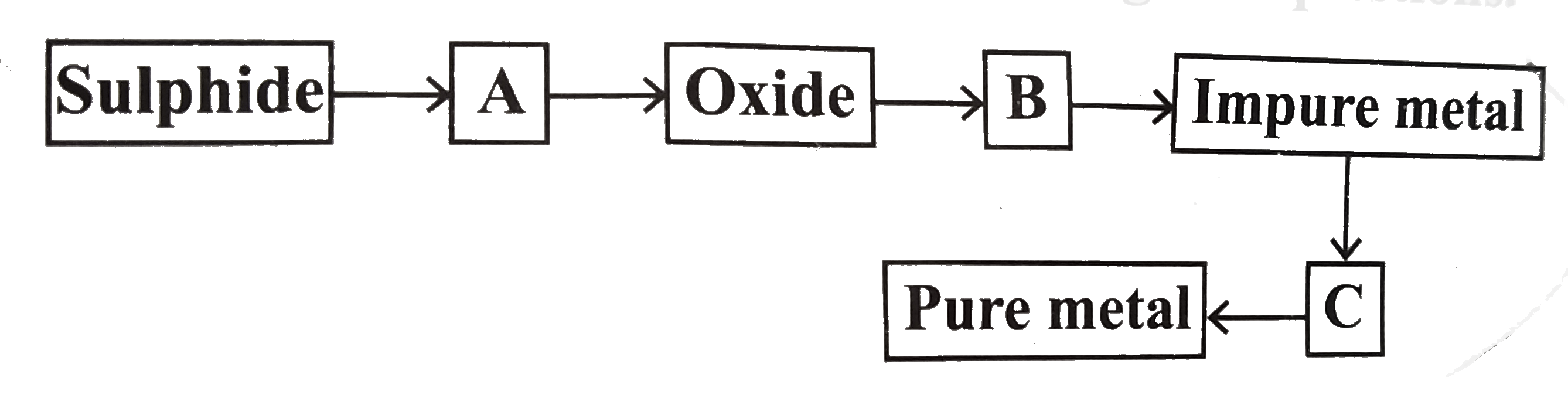
(ii) Step B (reduction) can be carried out ny using :
View SolutionThe metal extracted by electrolysis of its fused salt is:-
View SolutionWhich of the following metals are extracted by the electrolysis of their molten chlorides ?
View SolutionWhich of the following metal can be obtained by the electrolysis of the aqueous solution of their salts ?
View SolutionWhich one of the following metals can not be obtained on electrolysis of aqueous solution of its salts?
View Solution
