Graphite is used as anode but diamond is not.
There exist free electrons between two parallel sheets of graphite, hence it helps in electrode conduction.
If both (A) and ( R) are correct and ( R) is the correct explanation of (A).
If both (A) and ( R) are correct, but ( R) is not the correct explanation of (A).
If (A) is correct, but ( R) is incorrect.
If (A) is incorrect, but ( R) is correct.
The correct Answer is:A
Step-by-Step Solution:
1. Understanding the Question: The question states that graphite is used as an anode while diamond is not, and it asks for an explanation regarding this difference based on their electronic structures.
2. Analyzing Graphite:
- Graphite consists of carbon atoms arranged in layers of hexagonal structures.
- Each carbon atom in graphite is bonded to three other carbon atoms through covalent bonds, using three of its four valence electrons.
- The fourth electron from each carbon atom is free to move between the layers, creating a "sea of electrons."
3. Conductivity of Graphite:
- The presence of these free electrons allows graphite to conduct electricity effectively.
- The delocalization of these electrons between the layers enables graphite to act as a good conductor, similar to metals.
4. Analyzing Diamond:
- In contrast, diamond has a tetrahedral structure where each carbon atom is bonded to four other carbon atoms using all four of its valence electrons.
- This arrangement results in a strong covalent network with no free electrons available for conduction.
5. Conductivity of Diamond:
- Since all the electrons in diamond are involved in bonding, there are no free electrons to facilitate electrical conduction.
- Therefore, diamond cannot conduct electricity and is not suitable for use as an anode.
6. Conclusion:
- The assertion that graphite is used as an anode while diamond is not is correct because graphite has free electrons that allow it to conduct electricity, whereas diamond does not have free electrons due to its bonding structure.
7. Final Answer:
- The assertion is correct, and the reason provided (the existence of free electrons in graphite) is also correct and explains why graphite is used as an anode.
- Graphite consists of carbon atoms arranged in layers of hexagonal structures.
- Each carbon atom in graphite is bonded to three other carbon atoms through covalent bonds, using three of its four valence electrons.
- The fourth electron from each carbon atom is free to move between the layers, creating a "sea of electrons."
- The presence of these free electrons allows graphite to conduct electricity effectively.
- The delocalization of these electrons between the layers enables graphite to act as a good conductor, similar to metals.
- In contrast, diamond has a tetrahedral structure where each carbon atom is bonded to four other carbon atoms using all four of its valence electrons.
- This arrangement results in a strong covalent network with no free electrons available for conduction.
- Since all the electrons in diamond are involved in bonding, there are no free electrons to facilitate electrical conduction.
- Therefore, diamond cannot conduct electricity and is not suitable for use as an anode.
- The assertion that graphite is used as an anode while diamond is not is correct because graphite has free electrons that allow it to conduct electricity, whereas diamond does not have free electrons due to its bonding structure.
- The assertion is correct, and the reason provided (the existence of free electrons in graphite) is also correct and explains why graphite is used as an anode.
Topper's Solved these Questions
- GENERAL PRINCIPLES AND PROCESS OF ISOLATION OF ELEMENTSBOOK - CENGAGE CHEMISTRY ENGLISHCHAPTER - GENERAL PRINCIPLES AND PROCESS OF ISOLATION OF ELEMENTSEXERCISE - Exercise (Integer)
27 Videos
- GENERAL PRINCIPLES AND PROCESS OF ISOLATION OF ELEMENTSBOOK - CENGAGE CHEMISTRY ENGLISHCHAPTER - GENERAL PRINCIPLES AND PROCESS OF ISOLATION OF ELEMENTSEXERCISE - Exercise (Fill In The Blanks)
30 Videos
- GENERAL PRINCIPLES AND PROCESS OF ISOLATION OF ELEMENTSBOOK - CENGAGE CHEMISTRY ENGLISHCHAPTER - GENERAL PRINCIPLES AND PROCESS OF ISOLATION OF ELEMENTSEXERCISE - Exercise (Single Correcttype)
106 Videos
- ELECTROCHEMISTRYBOOK - CENGAGE CHEMISTRY ENGLISHCHAPTER - ELECTROCHEMISTRYEXERCISE - Archieves Subjective
35 Videos
- GRIGNARD REAGENTS AND ORGANOMETALLIC REAGENTSBOOK - CENGAGE CHEMISTRY ENGLISHCHAPTER - GRIGNARD REAGENTS AND ORGANOMETALLIC REAGENTSEXERCISE - Exercises Archives (Linked Comprehension)
1 Video
Similar Questions
Bauxite ore is concentrated by
View SolutionIron is the ____most abundant element in the earth's crust.
View SolutionThe impurities associated with the ore after mining are collectively called
View SolutionA current of 3.7 A is passed for 6hrs. Between Ni electrodes in 0.5 L of 2 M solution of Ni(NO3)2. What will be the molarity of solution at the end of electrolysis?
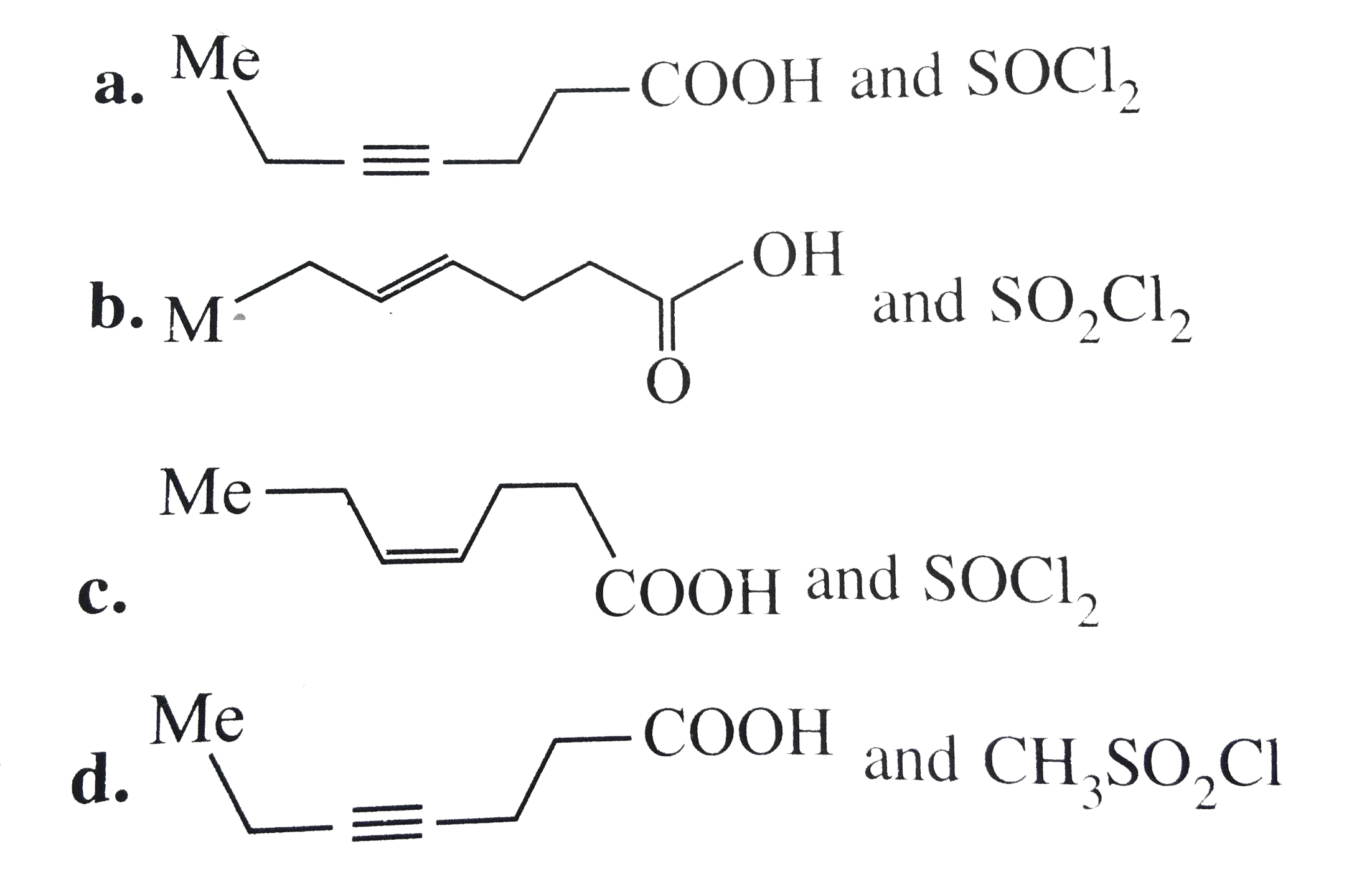
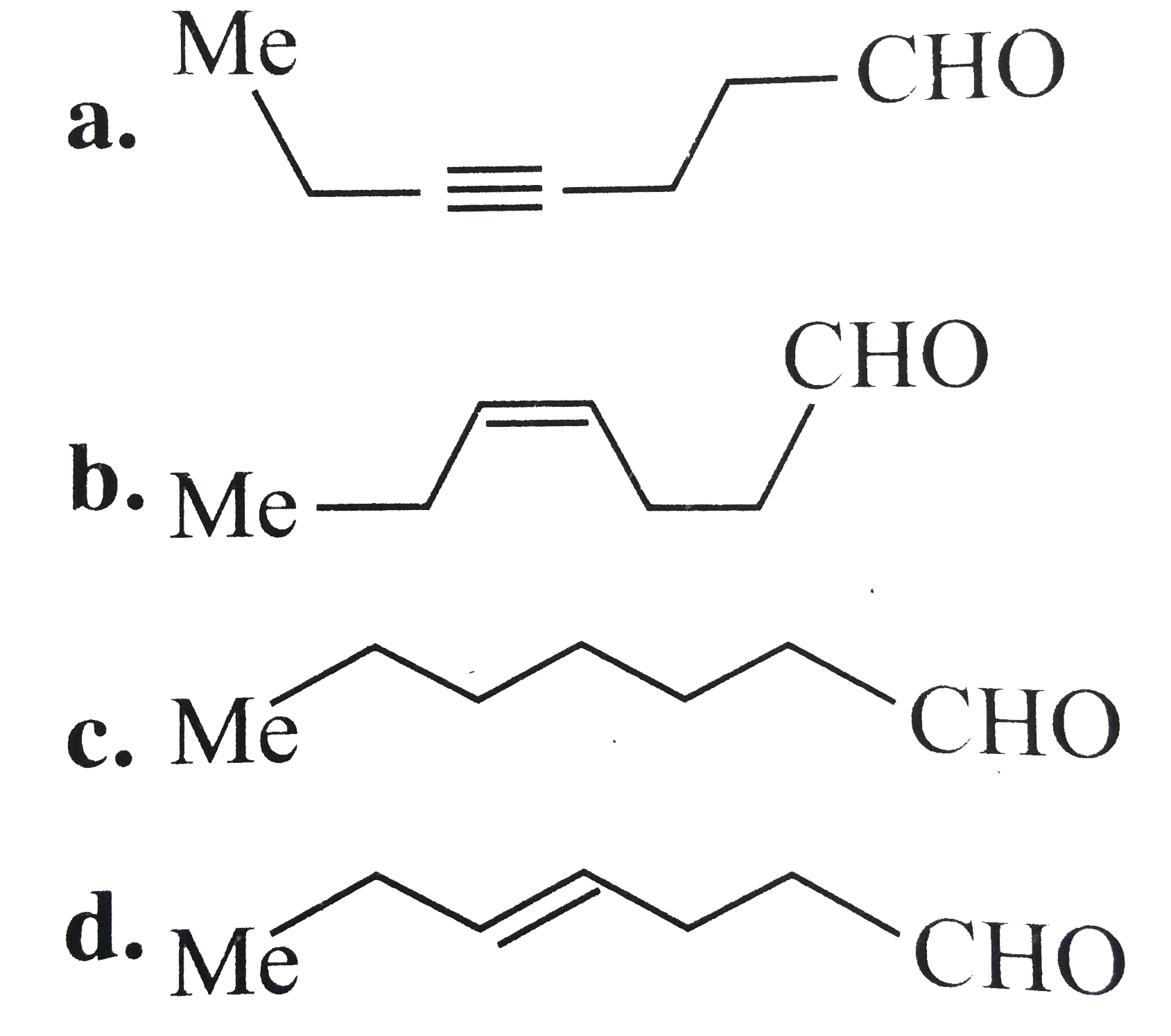
View SolutionIn the following reaction sequence, products I, J, and L are formed. K represents a reagent.
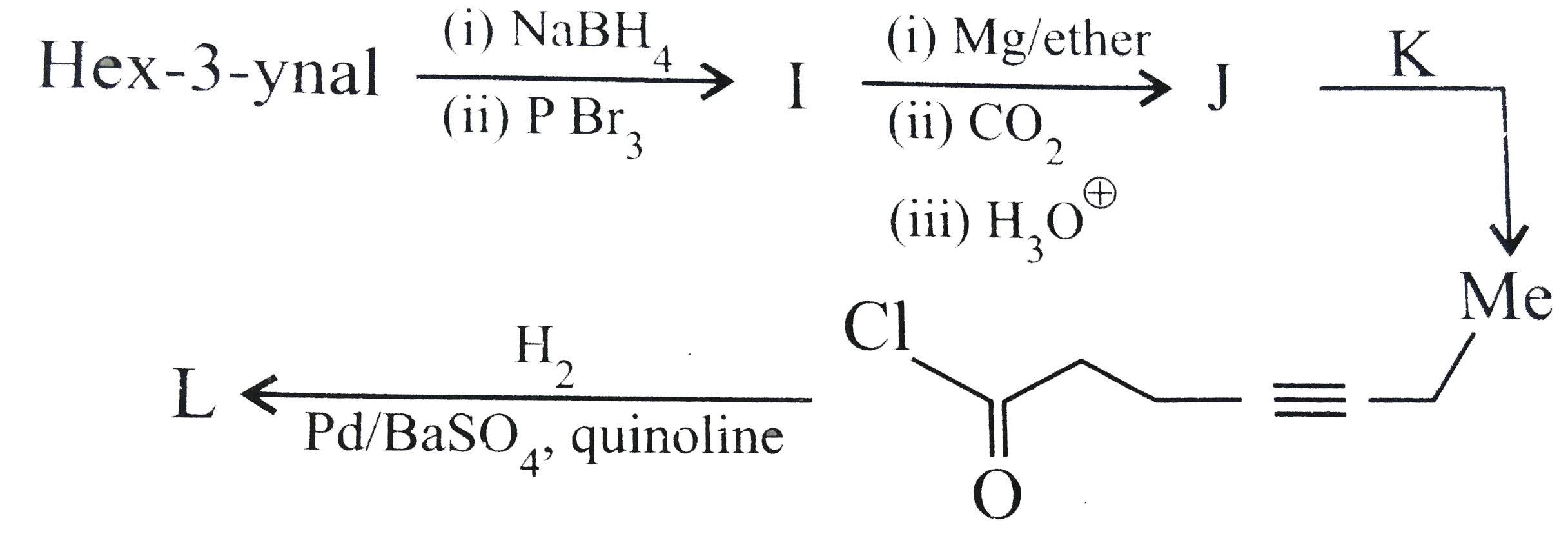
The structures of product I is :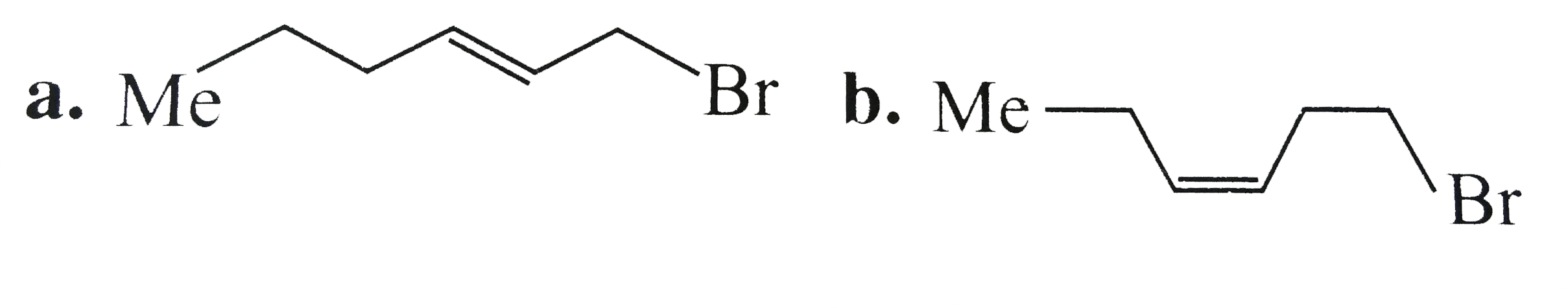
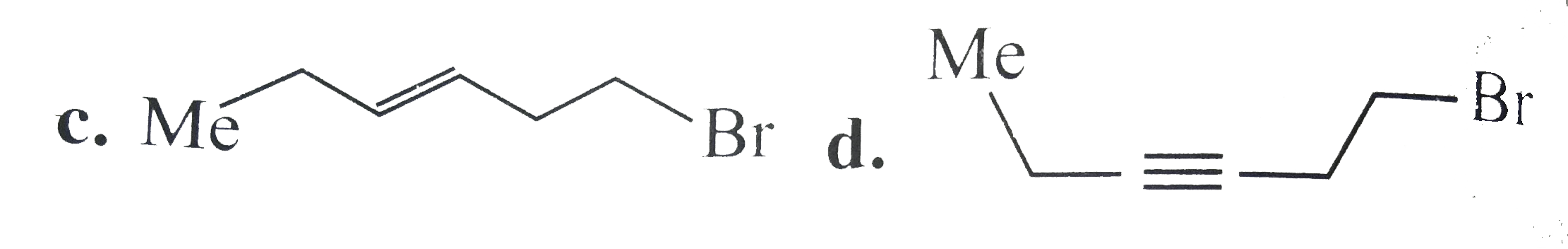
The structures of compounds J and K, respectively, are :
The structure of product L is :
View SolutionGraphite is used as anode but diamond is not.
There exist free electrons between two parallel sheets of graphite, hence it helps in electrode conduction.
View SolutionWith respect to graphite and diamond, which of the statements given below are correct? (i) Graphite is harder than diamond. (ii) Graphite has higher electrical conductivity than diamond. (iii) Graphite has higher thermal conductivity than diamond. Graphite has higher C-C bond order than diamond.
View SolutionWhy graphite is used as anode but not diamond ?
View SolutionStatement-1:Graphite is used as anode by not diamond.
Statement-2: Mobile electrons are present in graphite layer which help in the electrical conductivity.
View Solution(a) What is buckminsterfullerene? How is it related to diamond and graphite?
(b) Why is diamond used for making cutting tools (like glass cutters) but graphite is not?
(c) Why is graphite used for making dry cell electrodes but diamond is not?
View SolutionGraphite is used as anode and not diamond. Assign reason.
View Solutionडायमण्ड और ग्रेफाइट में अन्तर होता है
View SolutionGraphite is used as an anode but not diamond. Give reason.
View Solutionहीरा एवं ग्रेफाइट के उपयोग लिखिये।
View SolutionDistinguish between diamond and graphite.
View Solution

