Identify the compounds A, B, C, D, E and F in the following reactions :
E+Ca(OH)2→FWhiteprecipitate+H2O
E=CO2,F=CaCO3
Topper's Solved these Questions
- CARBON AND ITS COMPOUNDSBOOK - KUMAR PRAKASHANCHAPTER - CARBON AND ITS COMPOUNDSEXERCISE - Value Based Questions With Answers
12 Videos
- ACID , BASES AND SALTBOOK - KUMAR PRAKASHANCHAPTER - ACID , BASES AND SALTEXERCISE - Activity
47 Videos
- CHEMICAL REACTIONS AND EQUATIONS BOOK - KUMAR PRAKASHANCHAPTER - CHEMICAL REACTIONS AND EQUATIONS EXERCISE - PRACTICAL SKILL BASED QUESTIONS WITH ANSWERS
5 Videos
Similar Questions
One day Mudra was talking to her mother who was cooking vegetables in a stainless steel utensils. Mudra observed that the bottom of cooking utensils was getting blackened from outside. She showed this to her mother. The mother told Mudra that the bottoms of all the cooking utensils kept on the gas stove were getting blackened for the last few days and she had tough time cleaning these utensils. Being a science student of class X, Mudra checked the gas stove thoroughly and could understand the reason for this problem. She explained everything to her mother. As Mudra was getting late for school, she asked her mother to take a particular step to stop the blackening of cooking utensils. Mudra's mother did the same. The mother was glad that the bottoms of cooking utensils kept on gas burner were no longer being blackened.
Why is the bottom of cooking utensils getting blackened ? Explain.
View SolutionState the function of an indicator.
View SolutionWhat would happen if you add zinc coated iron nail into the solution of copper sulphate ? Give reason for your prediction.
View SolutionColemanite+Na2CO3Fused−−→(A)+(B)+CO2
(A)Solution+CO2→(B)+Na2CO3
(B)+concHCl→NaCl+(C)Acid
(C)Acid+H2O→(D)Acid
(D)Strong heating−−−−−−−−→(E)
(E)+CuSO4Heated−−−→inflame(F)Blue-coloured compound.
Compound (E) is.
View SolutionColemanite+Na2CO3Fused−−→(A)+(B)+CO2
(A)Solution+CO2→(B)+Na2CO3
(B)+concHCl→NaCl+(C)Acid
(C)Acid+H2O→(D)Acid
(D)Strong heating−−−−−−−−→(E)
(E)+CuSO4Heated−−−→inflame(F)Blue-coloured compound.
Compound (F) is.
View SolutionComplete the following reactions :
a.
b.
c.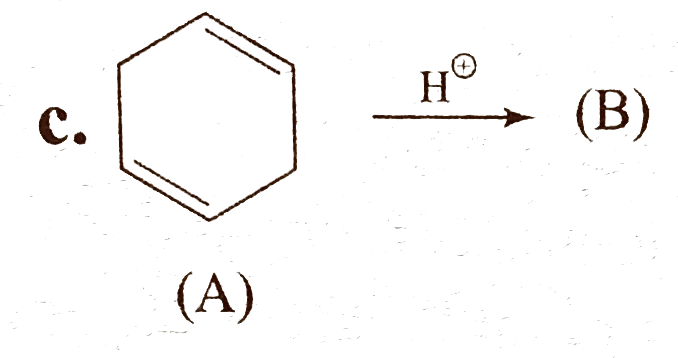
d.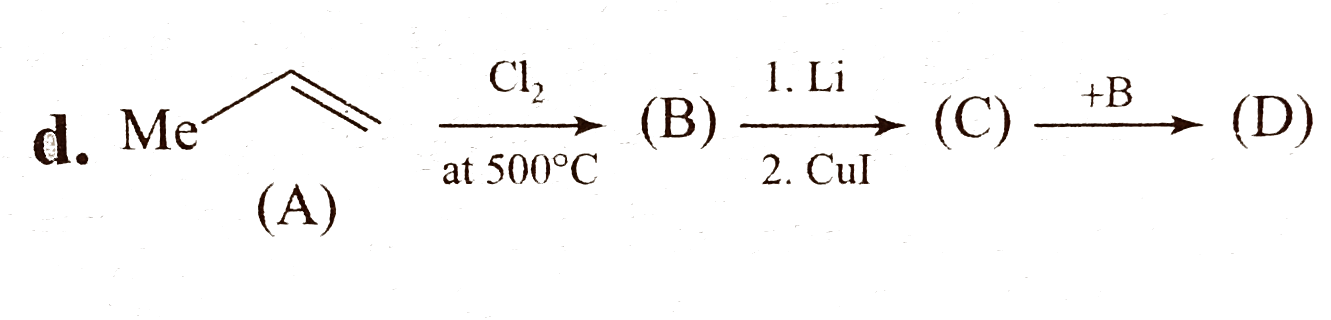
e.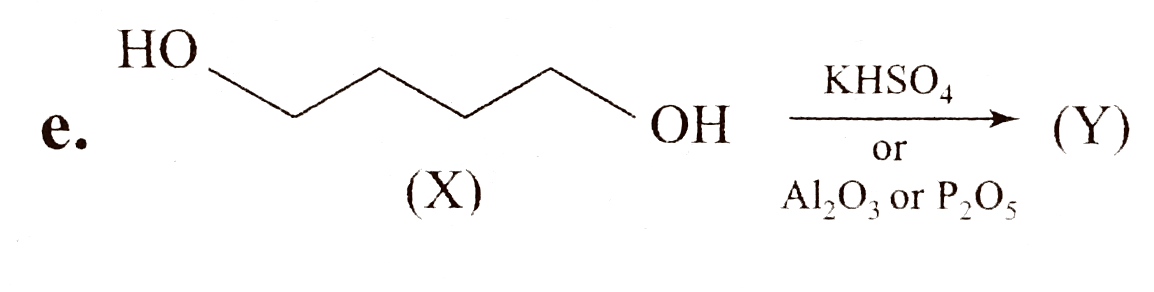
f.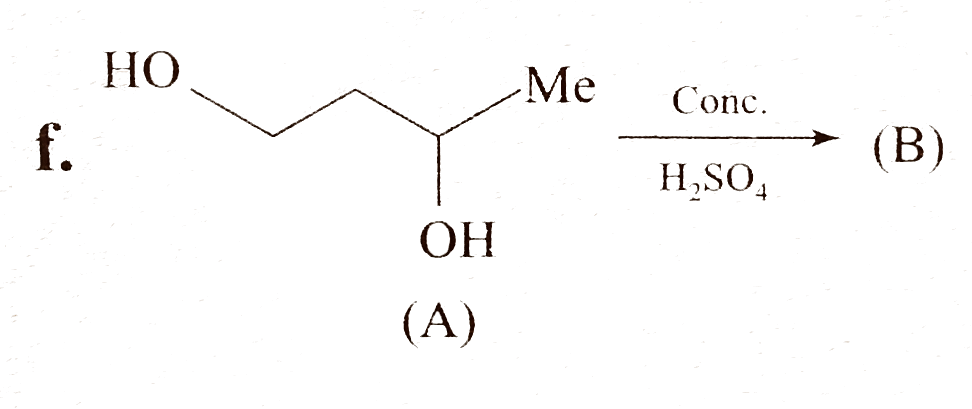
g.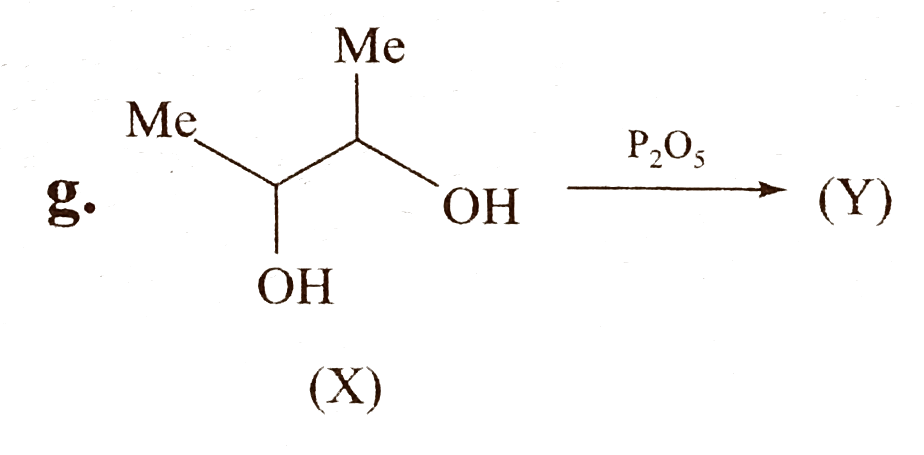 ltbr h.
ltbr h. 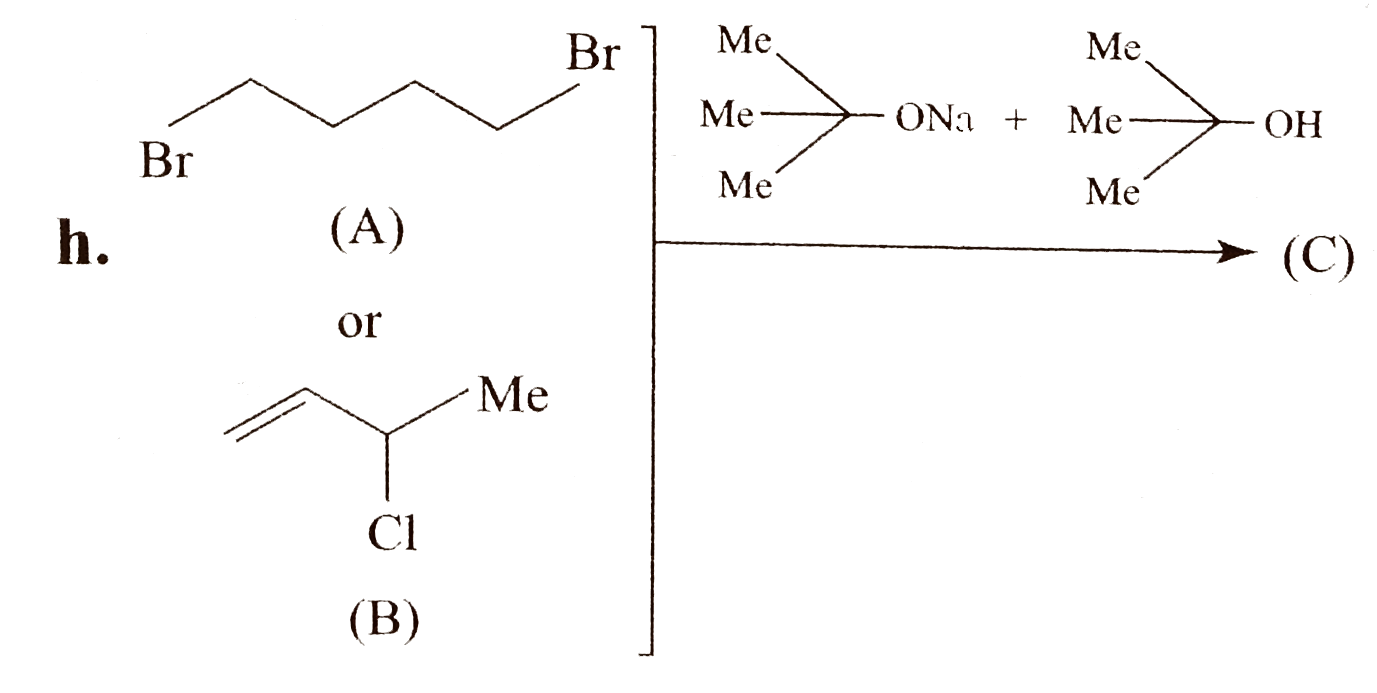
i.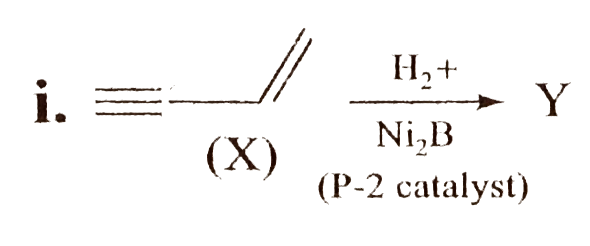
j.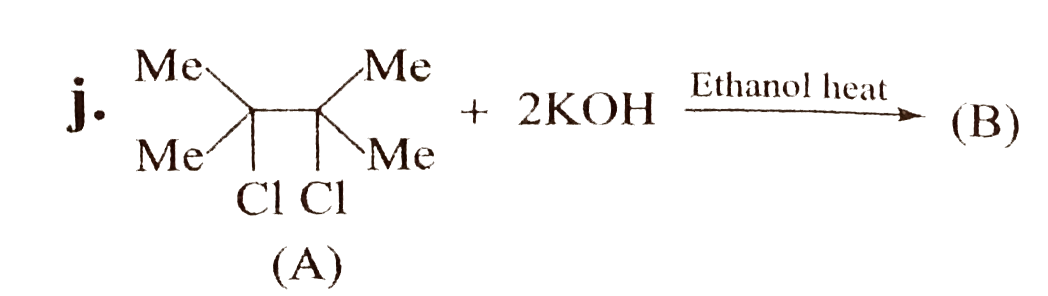
k.CH2=C=CH2(A)KNH2−−−−→orC2H5ONa+C2H5OH(B)
View SolutionComplete the following :
Mg(Air)−−−→heatcolourless solid[A]HCl−−→colourless solution[B]Na2CO3−−−−→white ppt[C]heat−−→colourless gas[D]Ca(OH)2−−−−−→white ppt[E]
View SolutionWhich of the following reagents will you choose for the given reactions?
H2C=CH2+H2O+O→CH2C∣OH−CH2∣OH
View SolutionFrom the following reactions. Identify A to F Mg overset("air"/Delta)(to)"Colourless solid" (A) overset(HCl)(to)"colourless solution"(B) overset(Na_(2)CO_(3))(to)"White ppt"(C )overset(Delta)(to)underset("white ppt"(F))underset(darrCa(OH)_(2))underset("gas")underset("colourless")((E))+(D)
View SolutionIdentify A-D in the following reactions . (1) A+B underset(Delta)overset("dil"NaOH) to C+H_(2)O (2) C overset("dil"NaOH) underset((ii) Zn//H_(2)O) to D+A (3)
View SolutionIdentify unknown compounds A to E in the following senes of chemical reactions. (i) (ii) A+B overset("dil NaOH")to C+ H_(2)O (iii) C overset((i) O_(3)) underset((ii) Zn//H_(2)O) to A+D (iv) D overset(H_(2)//Ni)underset(Delta) to E
View SolutionWhich is false for the following reaction ? underset( A)([Cu(H_(2)O)_(3) OH]^(+) )+underset(B) ( [ Al(H_(2)O)_(6)]^(3+))rarrunderset( C ) ([Cu(H_(2) O)_(4)]^(2+)) +underset( D) ( [Al(H_(2)O)_(5) OH]^(2+))
View SolutionIdentify the compounds A, B, C, D, E and F in the following reactions : CH_(3)CH_(2)OH+CH_(3)COOH overset("Acid")toB+H_(2)O
View SolutionIdentify the compounds A, B, C, D, E and F in the following reactions :
B+NaOH→C2H5OH+C
View SolutionIdentify the compounds A, B, C, D, E and F in the following reactions :
D+Na2CO3→CH3COONa+E+H2O
View Solution
