In the following diagram for the first three periods of the periodic table, five elements have been represented by the letters a,b,c,d and e (which are not their chemical symbols):

(i) Select the letter which represents a halogen.
(ii) Select the letter which represents a noble gas.
(iii) What type of bond is formed between a and b?
(iv) What type of bond is formed between c and b?
(v) Which element will form a divalent anion?
(i) The halogens are placed in group 17. Now, in the above given table the element present in group 17 is b. Thus, the letter b represents a halogen.
(ii) The noble gases are placed in group 18 of the periodic table. In the above given table, the element placed in group 18 is e. Thus, the letter e represents a noble gas.
(iii) We have now to find the type of bond formed betwen the elements a and b. Now, if we look at the above given table, we find that element a is placed non-metal reacts with another non-metal, the covalent bonds are formed. So, the bond between a and b is covalent bond.
(iv) And now we have to find out the type of bond between the elements c and b. The element c is in group 2 so it is a metal. The element b is in group 17 so it is a non metal. We know that when a metal reacts with a non -metal, then an ionic bond is formed. Thus, the bond formed between the elements c and b will be ionic bond.
(v) We will now find out that element which forms a divalent anion. A divalent means a negative ion having 2 units of negative charge. A divalent anion is formed by a non-metal atom having 6 valence electrons (so that it can accept 2 more electrons to complete the octet and form a divalent anion). Thus a divalent anion will be formed by a non metal element of group 16 because it will have 6 valence electrons in its atom. Now, in the above given table, the elemented has been placed in group 16 of the periodic table, so element will form a divalent anion.
Topper's Solved these Questions
- PERIODIC CLASSIFICATION OF ELEMENTSBook:LAKHMIR SINGH & MANJIT KAURChapter:PERIODIC CLASSIFICATION OF ELEMENTSExercise:LONG ANSWER TYPE
Explore 3 Videos
- PERIODIC CLASSIFICATION OF ELEMENTSBook:LAKHMIR SINGH & MANJIT KAURChapter:PERIODIC CLASSIFICATION OF ELEMENTSExercise:VERY SHORT ANSWER TYPE
Explore 6 Videos
- TEST PAPER 1Book:LAKHMIR SINGH & MANJIT KAURChapter:TEST PAPER 1Exercise:Section B
Explore 4 Videos
Similar Questions
(a) State Mendeleev's periodic law.
What chemical properties of elements were used by Mendeleev in creating his periodic table?
(c) State any three limitations of Mendeleev's classification of elements.
(d) Besides gallium, which two other elements have since been discovered for which Mendeleev had left gaps in the periodic table?
(e) Which group of elements was missing from Mendeleev's original periodic table?
View Solution(a) How does the chemical reactiviyt of alkali metals vary on going down in group 1 of the periodic table?
(b) How does the chemical reactivity of the halogens vary on going down in group 17 of the periodic table?
View Solution(a) A student dropped a few pieces of marble in dilute hydrochloric acid contained in
a test tube. The evolved gas was passed through lime water. What change would
be observed in lime water ? Write balanced chemical equations for both the
changes observed.
(b) State the chemical property in each case on which the following uses of baking
soda are based:
(i) as an antacid (ii) as a constituent of baking powder.
View SolutionIn the following table, six elements A, B, C, D, E and F of the modern periodic table with atomic numbers 3 to 18 are given: (a) Which of these is (i) Noble gas (ii) halogen? (b) Which of these is the most active metal in 3rd period? (c) Identify the most electronegative element in the third period. (d) In the compound between B and F what type of bond will be formed? (e) What would be the nature of oxide formed by C?
View SolutionIn the following table, six elements A, B, C, D, E and F (here letters are not the usual symbols of the elements) of the modern periodic table with their atomic numbers are given.
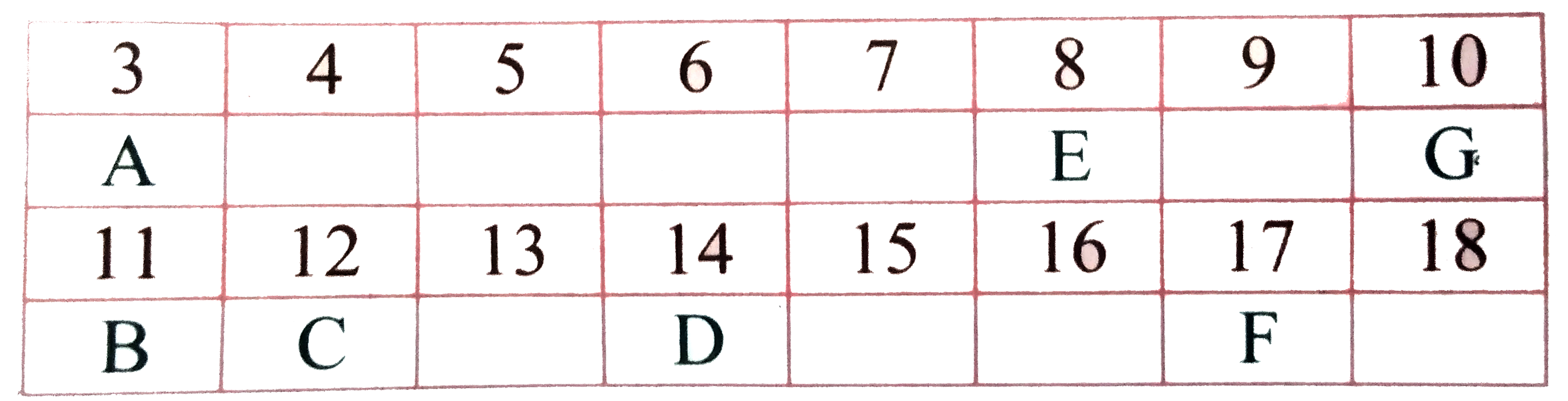
Which of these is an inert gas ?
View SolutionIn the following table, six elements A, B, C, D, E and F (here letters are not the usual symbols of the elements) of the modern periodic table with their atomic numbers are given. Which of these is a halogen ?
View SolutionIn the following table, six elements A, B, C, D, E and F (here letters are not the usual symbols of the elements) of the modern periodic table with their atomic numbers are given.
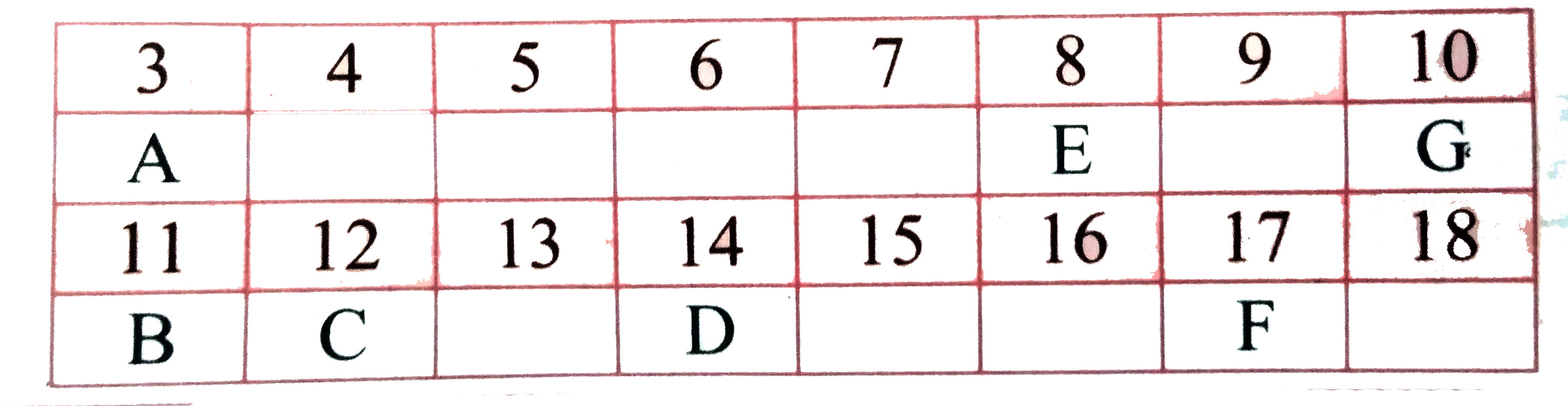
If B combines with F, what would be the formula of the compound formed?
View SolutionSome elements are given in the above table in their own symbol & position in the periodic table, while others [shaded] are shown with a letter. With reference to the table. In the compound between F and J, what type of bond will be formed
View Solution