In which C-C bond of CH3CH2CH2Br, the inductive effect is expected to be the least?
Text Solution
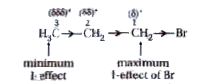
Magnitude of inductive effect diminishes as the number of intervening bonds increases. Hence, the effect is least in the bond between carbon -3 and hydrogen
|
Similar Questions
Knowledge Check
In which (C−C) bond of (H33C−2CH2−1CH2−Br), the inductive effect is expected to be the least ?
A1B2C3D2 and 3IUPAC name of CH3CH2C(Br)=CH−Cl is
A2-Bromo-1-chloro buteneB1-Chloro-2-bromo buteneC3-Chloro-2-bromo buteneDNone of the aboveThe IUPAC name of CH3CH2C(Br)=CHCl is
A2-bromo-1-chloro buteneB1-chloro-2-bromo buteneC3-chloro-2-bromo buteneDNone of the above
In CH3−CH2−CH2−Br,C−Br bond is formed by the overlapping of
View SolutionIn the compound
CH2=CH−CH2−CH2−C≡CH the C2−C3 bond is of
View SolutionIn CH3−CH2−CH2Br,C−Br bond is formed by the overlapping of __________.
View SolutionIn CH3−CH2−CH2−Br,C−Br bond is formed by the overlapping of
View SolutionIn the compound
CH2=CH−CH2−CH2−C≡CH the C2−C3 bond is of
View Solution

