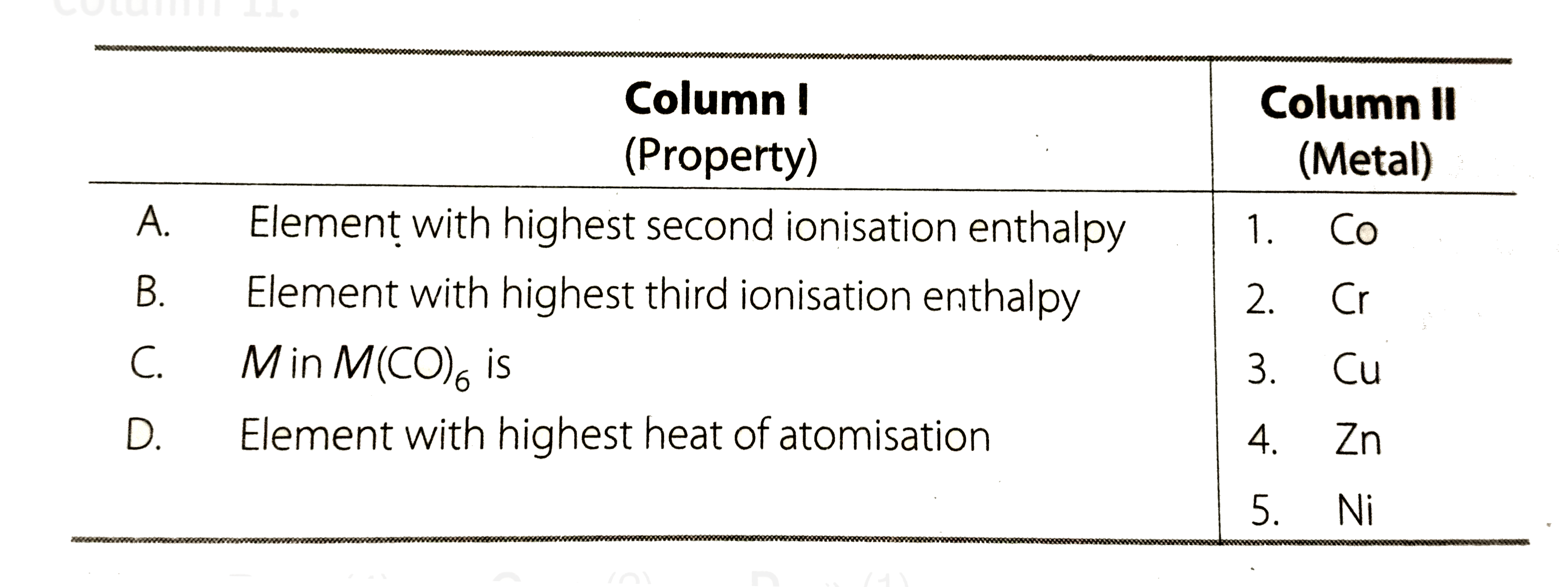Match the properties givens in Column I with the metals given in Column II.
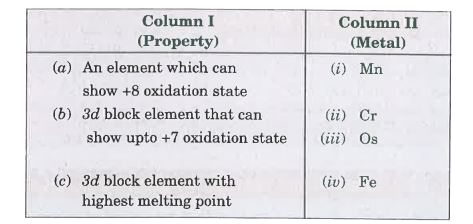
Column I ( Porperty)Column II(Metal) (i)An element which can show+8oxidation state(a)Mn(ii)3d block element that can show upto +7oxidation state(b)Cr(iii)3d block element with highest melting point(c)Os(d)Fe
The correct Answer is:(i)→(c),(ii)→(a),(iii)→(b)
To solve the matching question, we will analyze each property in Column I and identify the corresponding metal from Column II based on their oxidation states and properties.
1. Property (i): An element which can show +8 oxidation state
- Among the metals listed, Osmium (Os) is known to exhibit a +8 oxidation state. This is due to its ability to lose 8 electrons from its electronic configuration, which is (Xe) 4f14 5d6 6s2. Therefore, the correct match for this property is:
- (i) - (c) Os
2. Property (ii): 3d block element that can show up to +7 oxidation state
- Manganese (Mn) is the 3d block element that can show a +7 oxidation state. Its electronic configuration is (Ar) 3d5 4s2, and it can lose all 7 electrons (5 from 3d and 2 from 4s) to achieve this oxidation state. Thus, the correct match is:
- (ii) - (a) Mn
3. Property (iii): 3d block element with the highest melting point
- Chromium (Cr) has the highest melting point among the 3d block elements. Its electronic configuration is (Ar) 3d5 4s1, and it exhibits extra stability due to its half-filled d-subshell. Therefore, the correct match is:
- (iii) - (b) Cr
Final Matches:
- (i) - (c) Os
- (ii) - (a) Mn
- (iii) - (b) Cr
- Among the metals listed, Osmium (Os) is known to exhibit a +8 oxidation state. This is due to its ability to lose 8 electrons from its electronic configuration, which is (Xe) 4f14 5d6 6s2. Therefore, the correct match for this property is:
- (i) - (c) Os
- Manganese (Mn) is the 3d block element that can show a +7 oxidation state. Its electronic configuration is (Ar) 3d5 4s2, and it can lose all 7 electrons (5 from 3d and 2 from 4s) to achieve this oxidation state. Thus, the correct match is:
- (ii) - (a) Mn
- Chromium (Cr) has the highest melting point among the 3d block elements. Its electronic configuration is (Ar) 3d5 4s1, and it exhibits extra stability due to its half-filled d-subshell. Therefore, the correct match is:
- (iii) - (b) Cr
- (i) - (c) Os
- (ii) - (a) Mn
- (iii) - (b) Cr
Topper's Solved these Questions
- D- AND F-BLOCK ELEMENTSBook:PRADEEPChapter:D- AND F-BLOCK ELEMENTSExercise:ASSERTION AND REASON TYPE QUESTIONS
Explore 5 Videos
- D- AND F-BLOCK ELEMENTSBook:PRADEEPChapter:D- AND F-BLOCK ELEMENTSExercise:LONG ANSWER QUESTIONS
Explore 8 Videos
- D- AND F-BLOCK ELEMENTSBook:PRADEEPChapter:D- AND F-BLOCK ELEMENTSExercise:NCERT EXEMPLAR PROBLEMS(SHORT ANSWER QUESTIONS )
Explore 20 Videos
- CORDINATION COMPOUNDSBook:PRADEEPChapter:CORDINATION COMPOUNDSExercise:Important Questions
Explore 28 Videos
- ELECTROCHEMISTRYBook:PRADEEPChapter:ELECTROCHEMISTRYExercise:IMPORTANT QUESTIONS FOR BOARD EXAMINATION
Explore 25 Videos
Similar Questions
Knowledge Check
Metch the properties given in column I with the metals given in column II
 Ai−c,ii−d,iii−e,iv−aBi−c,ii−d,iii−b,iv−aCi−c,ii−d,iii−a,iv−eDi−d,ii−c,iii−a,iv−e
Ai−c,ii−d,iii−e,iv−aBi−c,ii−d,iii−b,iv−aCi−c,ii−d,iii−a,iv−eDi−d,ii−c,iii−a,iv−e
Match the properties given in Column I with the metals given in Column II.

View SolutionMatch the properties given in column I with the metals given in Column II.

View SolutionMatch the properties given in column I with the metals given in column II
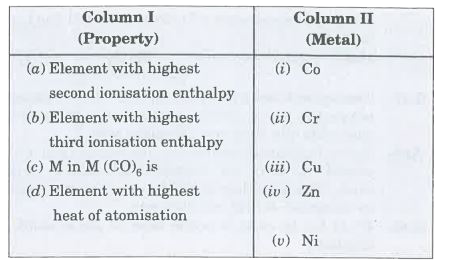
View SolutionMatch the properties given in column I with the metals given in column II
View SolutionMatch the properties given in column I with the element given in column II
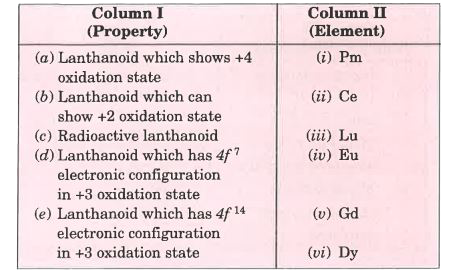
View SolutionMatch the property given in Column I with the element given in Column II.

View SolutionMatch the property given in Column I with the element given in Column II.

View Solution