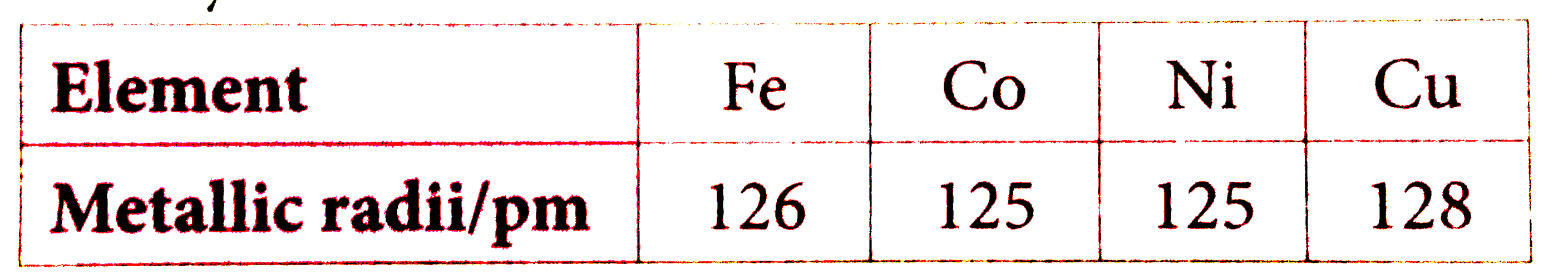Metallic radii of some transitions element are given below. Which of these elements will have highest density ?
ElementFeCoNiCuMetallic126125125128radii/pm
A
Fe
B
Ni
C
Co
D
Cu
Video Solution
Text Solution
The correct Answer is:D
(d) Copper has the highest density (8.95) sice its metallic radius is the minimum.
|
Similar Questions
Knowledge Check
Metallic radii of some transition elements are given below.
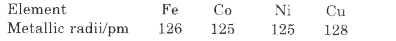
Which of these elements will have highest density ?AFeBNiCCoDCuMetallic radii of some transitions element are given below. Which of these elements will have highest density ?
ElementFeCoNiCuMetallic126125125128radii/pmAFeBNiCCoDCuThe transition elements are more metallic then p - block elements because they have
Aelectrons in d - orbitalsBelectron pairs in d- orbitalsCavailability of d - electrons for bondingDhigh ionisation energy
The atomic numbers of four elements are given below. Which one is an alkaline earth metal?
View SolutionThe transition elements are more metallic than the representative elements because they have
View SolutionThe transition elements are more metallic than the representative elements because they have
View SolutionThe atomic radii of transition elements from Cr to Cu are almost equal because
View SolutionThe electronic configuration of the elements X,Y, Z and J are given below . Which element has the highest metallic character
View Solution