Observe the given table and question: answer the following
ElementsABCDEAtomic number1142719
Identify the two elements that belong to the same period and the two elements that belong to the same group. Give reason for your conclusion. Solution in Kannada
The electronic configurations of the given elements are: A (11) = (2, 8, 1), B (4) =(2, 2), C (2) = (2), D (7) = (2,5), E (19) = (2, 8, 8, 1). B and D belong to same period since they have two shells each. A and E belong to same group since they have same number of valence electrons, i.e., I.
Similar Questions
Given below are some elements of the Modern Periodic Table. Atomic number of the element is given in the parentheses:
A(4), B(9), C(14), D(19), E(20)
(a) Select the element that has one electron in the outermost shell. Also write the electronic configuration of this element.
(b) Which two elements amongst these belong to the same group? Give reason for your answer.
(c) Which two elements amongst these belong to the same period? Which one of the two has bigger atomic radius?
View SolutionFour elements A, B, C and D along with their electronic configuration are given below: Now answer the following questions: (i) Which two elements belong to the same period ? (ii) Which two elements belong to the same group ? (iii) Which element out of A and C is more reactive and why?
View SolutionWhich of the given elements A,B,C,D and E with atomic number 2,3,7,10 and 30 respectively belong to the same period ?
View SolutionThe atoms of elements belonging to the same group of periodic table have the same
View Solutionतत्व A,B,C,D तथा E के परमाणु क्रमांक क्रमश : 9,11,17,12 तथा 13 हैं। तत्वों का कौन-सा युग्म समान समूह से संबंधित हैं ?
View SolutionObserve the given table and answer the following question :

Identify the two elements that belong to the same period and the two elements that belong to the same group. Give reason for your conclusion.
View SolutionAtoms of two different elements are represented in the following diagram.
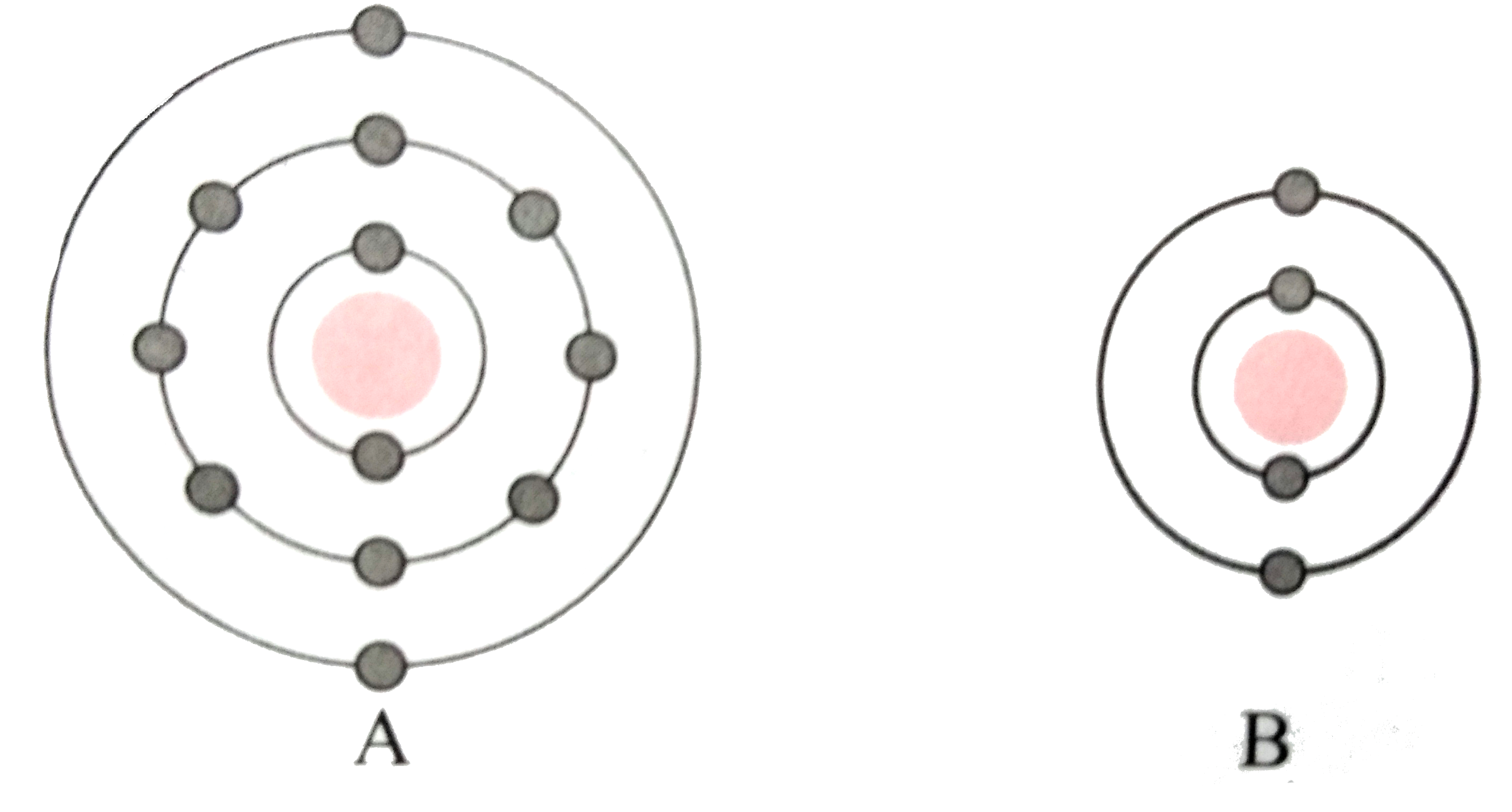
Do these elements belong to the same group ? Justify your answer.
View Solution
