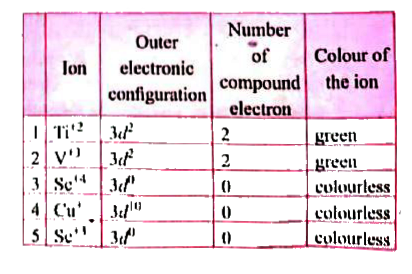
Predict which of the following will be coloured in aqueous solution Ti2+,V3+,Sc4+,Cu+,Sc3+,Fe3+,Ni2+ and Co3+.
These ions which have incompletely filled .d. orbitals will be coloured. These with fully filled or empty .d. orbitals are colourless.
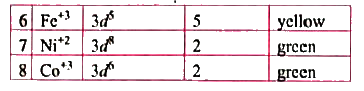
Topper's Solved these Questions
- TRANSITION AND INNER TRANSITION ELEMENTS BOOK - PREMIERS PUBLISHERSCHAPTER - TRANSITION AND INNER TRANSITION ELEMENTS EXERCISE - OTHER IMPORTANT QUESTIONS & ANSWERS (I. Choose the correct answer)
27 Videos
- TRANSITION AND INNER TRANSITION ELEMENTS BOOK - PREMIERS PUBLISHERSCHAPTER - TRANSITION AND INNER TRANSITION ELEMENTS EXERCISE - OTHER IMPORTANT QUESTIONS & ANSWERS (II. Answer the following questions)
114 Videos
- TRANSITION AND INNER TRANSITION ELEMENTS BOOK - PREMIERS PUBLISHERSCHAPTER - TRANSITION AND INNER TRANSITION ELEMENTS EXERCISE - OTHER IMPORTANT QUESTIONS & ANSWERS (II. Answer the following questions)
114 Videos
- SURFACE CHEMISTRYBOOK - PREMIERS PUBLISHERSCHAPTER - SURFACE CHEMISTRYEXERCISE - OTHER IMPORTANT QUESTIONS & ANSWERS (II. Answer the following questions:)
57 Videos
Similar Questions
The correct order of decreasing second ionisation enthalpy of Ti(22), V(23), Cr(24) and Mn(25) is :
View SolutionWrite the electronic configuration of (a) Cr+3, (b) Cu+, (c ) CO+2, (d) Mn+2, (e ) Pm+3, (f) Ce+4, (g) Lu+2, (h) Th+4.
View SolutionWrite the electronic configuration of (a) Cr+3, (b) Cu+, (c ) CO+2, (d) Mn+2, (e ) Pm+3, (f) Ce+4, (g) Lu+2, (h) Th+4.
View SolutionGive three examples of adsorption
View SolutionPredict which of the followingwill be coloured in aqueous solution? Ti3+,V3+,Cu⊕,Sc3+,Mn2+,Fe2+ and Co2+ Give reasons for each.
View SolutionPredict which of the following will be coloured in aqueous solution ? Ti^(3+), V^(3+),Cu^(+),Sc^(3+), Mn^(2+), Fe^(3+), Co^(2+) .
View SolutionPredict which of the following will be coloured in aqueous solution ?
Ti3+,V3+,Sc3+,Mn2+,Fe3+,Co2+ and MnO−4
View SolutionPredict which of he ions Cu+,Sc3+,Mn2+,Fe2+ are coloured in aqueous solution ? Give reasons.
View SolutionPredic which of the following will coloured in aqueous solution Ti^(2+) , V^(3+) Sc^(4+) Cu^(+) ,Sc^(3+) ,Fe^(3+) ,Ni^(2+) and Co^(3+)
View SolutionWhich of the following ions will be coloured in aqueous solution ? Ti^(3+), V^(3+), MnO_(4)^(-), Sc^(3+), Mn^(2+), Fe^(3+), Co^(2+) .
View SolutionPredict which of the following will be coloured in aqueous solution ? Ti3+,V3+,Cu+,Sc3+,Mn2+,Fe3+ and Co2+. Give reasons for each.
View SolutionTi3+,V3+,Mn2+,Fe3+,Co2+ form coloured aqueous solutions but Sc3+,Cu+ do not - explain.
View SolutionWhich of the following are coloured in aqueous solution ? Give reasons : V^(3+), Cu^(+) , Sc^(3+) ,Fe^(3+)
View SolutionPredict which of the following will be coloured in aqueous solution? Ti^(3+), V^(3+), Cu^(+), Sc^(3+), Mn^(2+), Fe^(3+) and Co^(2+) . Give reasons for each
View SolutionPredict which of the following will be coloured in aqueous solution Ti^(2+), V^(3+), Se^(4+), Cu^(+), Sc^(3+), Fe^(3+), Ni^(2+) , and Co^(3+)
View SolutionPredict which of the following will be coloured in aqueous solution ?
Ti3+,V3+,Cu+,Sc3+,Mn2+,Fe3+ and Co2+.
Give reasons for each.
View Solution

