Quantitative estimation of C,H and extra elements (e.g., N.S.P., and halogens) is carried out by Liebig's combustion, Carius, Dumas, and Kjeldahl's method.
Q. In the quantitative estimation of phosphorous by using ammonium molybdate, the formula used is: Where W is the mass of ammonium phosphomolybdate and w is the mass of the compound.
Percentage of P=311877×W×100w
Percentage of P=621877×W×100w
Percentage of P=311877×w×100W
Percentage of P=621877×w×100W
The correct Answer is:A
To solve the problem of quantitative estimation of phosphorus using ammonium molybdate, we can follow these steps:
1. Understanding the Components:
- We need to estimate phosphorus (P) in a compound using ammonium phosphomolybdate.
- The formula for ammonium phosphomolybdate is NH4PO4⋅12MoO3.
2. Identify the Molar Mass:
- The molar mass of ammonium phosphomolybdate (NH4)3PO4·12MoO3 is given as 1877 g/mol.
- The molar mass of phosphorus (P) is 31 g/mol.
3. Setting Up the Formula:
- The percentage of phosphorus in the compound can be calculated using the formula:
Percentage of Phosphorus=(311877)×Ww×100
- Where:
- W = mass of ammonium phosphomolybdate
- w = mass of the compound being analyzed
4. Final Formula:
- Thus, the final formula for calculating the percentage of phosphorus is:
Percentage of Phosphorus=31×W1877×w×100
5. Conclusion:
- The answer to the question is that the formula used for the quantitative estimation of phosphorus by using ammonium molybdate is:
Percentage of Phosphorus=311877×W×100÷w
- This corresponds to option number 1.
- We need to estimate phosphorus (P) in a compound using ammonium phosphomolybdate.
- The formula for ammonium phosphomolybdate is NH4PO4⋅12MoO3.
- The molar mass of ammonium phosphomolybdate (NH4)3PO4·12MoO3 is given as 1877 g/mol.
- The molar mass of phosphorus (P) is 31 g/mol.
- The percentage of phosphorus in the compound can be calculated using the formula:
Percentage of Phosphorus=(311877)×Ww×100
- Where:
- W = mass of ammonium phosphomolybdate
- w = mass of the compound being analyzed
- Thus, the final formula for calculating the percentage of phosphorus is:
Percentage of Phosphorus=31×W1877×w×100
- The answer to the question is that the formula used for the quantitative estimation of phosphorus by using ammonium molybdate is:
Percentage of Phosphorus=311877×W×100÷w
- This corresponds to option number 1.
Topper's Solved these Questions
- PURIFICATION OF ORGANIC COMPOUNDS AND QUALITATIVE AND QUANTITATIVE ANALYSISBook:CENGAGE CHEMISTRY ENGLISHChapter:PURIFICATION OF ORGANIC COMPOUNDS AND QUALITATIVE AND QUANTITATIVE ANALYSISExercise:Multiple Correct Answers Type
Explore 10 Videos
- PURIFICATION OF ORGANIC COMPOUNDS AND QUALITATIVE AND QUANTITATIVE ANALYSISBook:CENGAGE CHEMISTRY ENGLISHChapter:PURIFICATION OF ORGANIC COMPOUNDS AND QUALITATIVE AND QUANTITATIVE ANALYSISExercise:Single Correct Answer Type
Explore 40 Videos
- PURIFICATION OF ORGANIC COMPOUNDS AND QUALITATIVE AND QUANTITATIVE ANALYSISBook:CENGAGE CHEMISTRY ENGLISHChapter:PURIFICATION OF ORGANIC COMPOUNDS AND QUALITATIVE AND QUANTITATIVE ANALYSISExercise:Concept Application Type
Explore 23 Videos
- PERIODIC CLASSIFICATION OF ELEMENTS AND GENERAL INORGANIC CHEMISTRYBook:CENGAGE CHEMISTRY ENGLISHChapter:PERIODIC CLASSIFICATION OF ELEMENTS AND GENERAL INORGANIC CHEMISTRYExercise:Exercises (Archives )Subjective
Explore 4 Videos
- REDOX REACTIONSBook:CENGAGE CHEMISTRY ENGLISHChapter:REDOX REACTIONSExercise:Archives (Integers)
Explore 1 Video
Similar Questions
Which of the following statements is/are correct?
View SolutionForty millililtre of CO was mixed with 100 ml of O2 and the mixture was exploded. On cooling, the reaction mixture was shaken with KOH What volume of gas is left?
View SolutionGive a brief description of the principles of the following techniques taking an example in each case.
(a) Crystallisation (b) Distillation (c) Chromatography
View SolutionArrange the following in the given order
(a) Decreasing ionic size, Mg2+,O2−,Na⊕,F⊕
(b) Increasing bond length F2,N2,Cl2O2
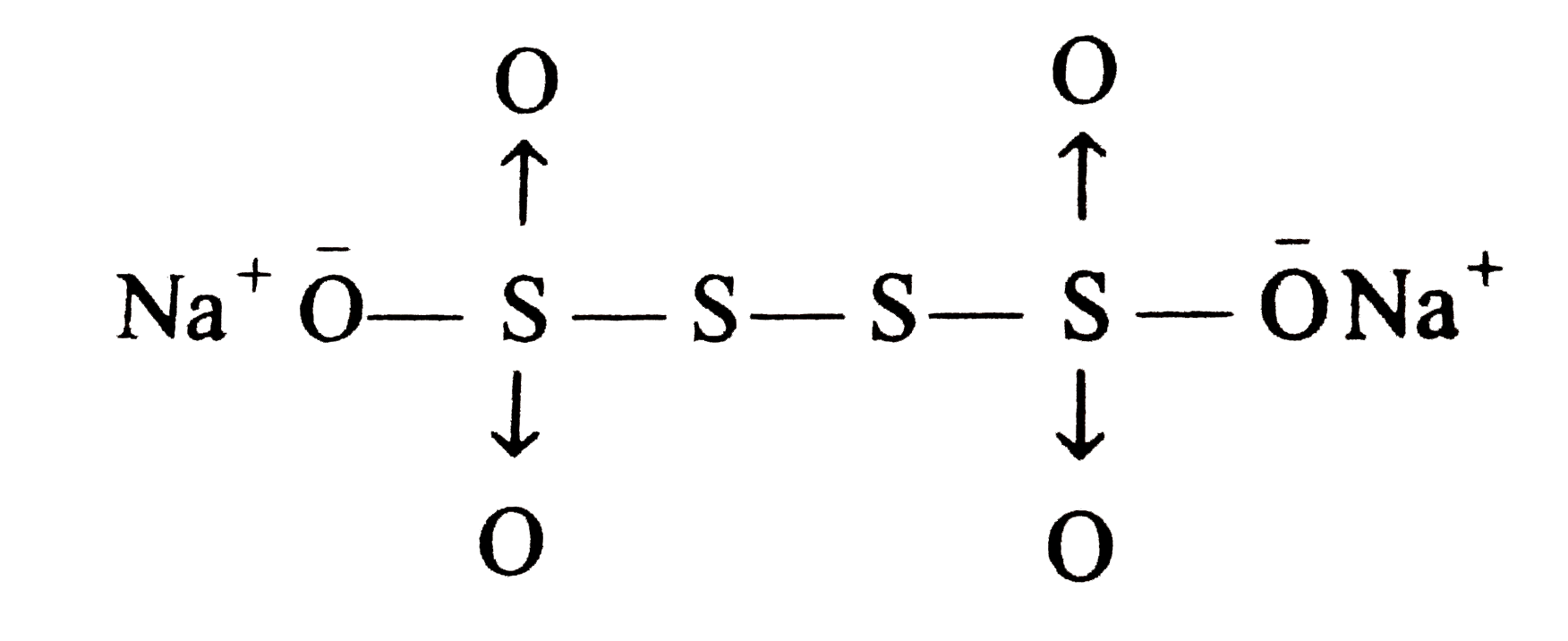
View SolutionThe difference in the oxidation numbers of the two types of sulphur atoms in Na2S4O6 is:
View SolutionQuantitative estimatin of C,H and extra elements (e.g., N.S.P., and halogens) is carried out by Liebig's combustion, Carius, Dumas, and Kjeldahl's method. Q. Liebig's combustion method is used for the quantitative estimation of :
View SolutionQuantitative estimatin of C,H and extra elements (e.g., N.S.P., and halogens) is carried out by Liebig's combustion, Carius, Dumas, and Kjeldahl's method. Q. Carius method is used for the quantitative estimation of:
View SolutionQuantitative estimatin of C,H and extra elements (e.g., N.S.P., and halogens) is carried out by Liebig's combustion, Carius, Dumas, and Kjeldahl's method.
Q. Dumas and Kjeldahl's method are used for the quantitative estimation of
View SolutionQuantitative estimatin of C,H and extra elements (e.g., N.S.P., and halogens) is carried out by Liebig's combustion, Carius, Dumas, and Kjeldahl's method. Q. In carius method for the quantitative estimation of phosphorous by using magnesia mixture, phosphorous is estimated by:
View SolutionQuantitative estimatin of C,H and extra elements (e.g., N.S.P., and halogens) is carried out by Liebig's combustion, Carius, Dumas, and Kjeldahl's method.
Q. In Carius method for the quantitative estimation of sulphur, it is estimated by:
View SolutionQuantitative estimatin of C,H and extra elements (e.g., N.S.P., and halogens) is carried out by Liebig's combustion, Carius, Dumas, and Kjeldahl's method. Q. Carius is the name of:
View SolutionQuantitative estimatin of C,H and extra elements (e.g., N.S.P., and halogens) is carried out by Liebig's combustion, Carius, Dumas, and Kjeldahl's method. Q. Kjeldagl's is the name of
View SolutionQuantitative estimatin of C,H and extra elements (e.g., N.S.P., and halogens) is carried out by Liebig's combustion, Carius, Dumas, and Kjeldahl's method.
Q. In the quantitative estimation of phosphorous by using magnesia mixture, the formula used is: Where W is the mass of Mg2P2O7 and w is the mass of the compound.
View SolutionQuantitative estimatin of C,H and extra elements (e.g., N.S.P., and halogens) is carried out by Liebig's combustion, Carius, Dumas, and Kjeldahl's method.
Q. In the quantitative estimation of phosphorous by usin ammonium molybdate, the formula used is: Where W is the mass of ammonium phospho molybdate and w is the mass of the compound.
View SolutionQuantitative estimatin of C,H and extra elements (e.g., N.S.P., and halogens) is carried out by Liebig's combustion, Carius, Dumas, and Kjeldahl's method.
Q. In the quntitative estimation of oxygen by using I2O5. The formula used is:
Where W is the mass of CO2 and w is the mass of the compound.
View SolutionQuantitative estimatin of C,H and extra elements (e.g., N.S.P., and halogens) is carried out by Liebig's combustion, Carius, Dumas, and Kjeldahl's method. Q. Liebig's combustion method is used for the quantitative estimation of :
View Solution
