Questions given below are based on the given diagram for extractive metallurgy.
(iv) At 1100∘C, which reaction is spontaneous to a maximum extent ?
MgO+C→Mg+CO
ZnO+C→Zn+CO
MgO+Zn→Mg+ZnO
ZnO+Mg→MgO+Zn
The correct Answer is:D
To determine which reaction is spontaneous to a maximum extent at 1100°C, we need to analyze the given reactions based on their Gibbs free energy (ΔG). A reaction is considered spontaneous if ΔG is negative.
1. Understand the Concept of Spontaneity:
- A reaction is spontaneous when ΔG < 0. This indicates that the reactants can convert to products without the need for external energy.
2. Analyze Each Reaction:
- We will evaluate the given options one by one to see which reaction has the most negative ΔG.
3. Option A: Magnesium Oxide and Carbon:
- The reaction involves magnesium oxide (MgO) and carbon (C) producing carbon monoxide (CO) and magnesium (Mg).
- Here, carbon is reducing magnesium oxide. However, magnesium has a more negative ΔG than carbon, meaning magnesium can reduce carbon, not the other way around. Thus, this reaction is not spontaneous.
4. Option B: Zinc Oxide and Carbon:
- This reaction involves zinc oxide (ZnO) and carbon producing zinc (Zn) and carbon monoxide (CO).
- In this case, zinc is reducing carbon monoxide. However, since zinc is less reactive than carbon, this reaction is also not spontaneous.
5. Option C: Magnesium Oxide Formation:
- This option involves magnesium oxide (MgO) and zinc (Zn) where zinc is acting as a reducing agent.
- Since magnesium is below zinc in the reactivity series, magnesium cannot reduce zinc oxide. Therefore, this reaction is not spontaneous.
6. Option D: Magnesium and Zinc Oxide:
- This reaction involves magnesium (Mg) and zinc oxide (ZnO) producing zinc (Zn) and magnesium oxide (MgO).
- Here, magnesium is reducing zinc oxide to zinc. Since magnesium has a more negative ΔG compared to zinc, this reaction is spontaneous to a maximum extent.
7. Conclusion:
- The reaction that is spontaneous to a maximum extent at 1100°C is the one where magnesium reduces zinc oxide to zinc.
Final Answer:
The reaction where magnesium reduces zinc oxide to zinc (Option D) is spontaneous to a maximum extent at 1100°C.
- A reaction is spontaneous when ΔG < 0. This indicates that the reactants can convert to products without the need for external energy.
- We will evaluate the given options one by one to see which reaction has the most negative ΔG.
- The reaction involves magnesium oxide (MgO) and carbon (C) producing carbon monoxide (CO) and magnesium (Mg).
- Here, carbon is reducing magnesium oxide. However, magnesium has a more negative ΔG than carbon, meaning magnesium can reduce carbon, not the other way around. Thus, this reaction is not spontaneous.
- This reaction involves zinc oxide (ZnO) and carbon producing zinc (Zn) and carbon monoxide (CO).
- In this case, zinc is reducing carbon monoxide. However, since zinc is less reactive than carbon, this reaction is also not spontaneous.
- This option involves magnesium oxide (MgO) and zinc (Zn) where zinc is acting as a reducing agent.
- Since magnesium is below zinc in the reactivity series, magnesium cannot reduce zinc oxide. Therefore, this reaction is not spontaneous.
- This reaction involves magnesium (Mg) and zinc oxide (ZnO) producing zinc (Zn) and magnesium oxide (MgO).
- Here, magnesium is reducing zinc oxide to zinc. Since magnesium has a more negative ΔG compared to zinc, this reaction is spontaneous to a maximum extent.
- The reaction that is spontaneous to a maximum extent at 1100°C is the one where magnesium reduces zinc oxide to zinc.
The reaction where magnesium reduces zinc oxide to zinc (Option D) is spontaneous to a maximum extent at 1100°C.
Topper's Solved these Questions
- GENERAL PRINCIPLES AND PROCESS OF ISOLATION OF ELEMENTSBOOK - CENGAGE CHEMISTRY ENGLISHCHAPTER - GENERAL PRINCIPLES AND PROCESS OF ISOLATION OF ELEMENTSEXERCISE - Exs 1.1 (Subjective)
25 Videos
- GENERAL PRINCIPLES AND PROCESS OF ISOLATION OF ELEMENTSBOOK - CENGAGE CHEMISTRY ENGLISHCHAPTER - GENERAL PRINCIPLES AND PROCESS OF ISOLATION OF ELEMENTSEXERCISE - Exs 1.1 (Objective)
14 Videos
- GENERAL PRINCIPLES AND PROCESS OF ISOLATION OF ELEMENTSBOOK - CENGAGE CHEMISTRY ENGLISHCHAPTER - GENERAL PRINCIPLES AND PROCESS OF ISOLATION OF ELEMENTSEXERCISE - Archives (Subjective)
14 Videos
- ELECTROCHEMISTRYBOOK - CENGAGE CHEMISTRY ENGLISHCHAPTER - ELECTROCHEMISTRYEXERCISE - Archieves Subjective
35 Videos
- GRIGNARD REAGENTS AND ORGANOMETALLIC REAGENTSBOOK - CENGAGE CHEMISTRY ENGLISHCHAPTER - GRIGNARD REAGENTS AND ORGANOMETALLIC REAGENTSEXERCISE - Exercises Archives (Linked Comprehension)
1 Video
Similar Questions
Questions given below are based on the given diagram for extractive metallurgy.
(v) This method is known as :
View SolutionQuestions given below are based on the given diagram for extractive metallurgy.
(iii) The make the following process spontaneous, temperature should be :
ZnO+C→Zn+CO.
View Solution
Knowledge Check
Which energy diagram best represents the given reaction ?
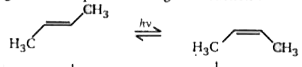 A
A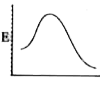 B
B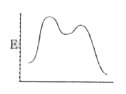 C
C D
D
Questions given below are based on the given diagram for extractive metallurgy.
(i) At what approximate temperature, zinc and carbon have equal affinity for oxygen ?
View SolutionFree enegry , G=H−TS, is state function that indicates whther a reaction is spontaneous or non-spontaneous. If you think of TS as the part of the system's enegry that is disordered already, then (H−TS) is the part of the system's energy that is still ordered and therefore free to cause spontaneous change by becoming disordered.
Also, ΔG=ΔH−TΔS From the second law of thermodynamics, a reaction is spontaneous if ΔtotalS is positive, non-spontaneous if ΔtotalS is negative, and at equilibrium if Δ'→talS is zero.
Since, −TΔS=ΔG and since ΔG and ΔS have opposite sings, we can restate the thermodynamic criterion for the spontaneity of a reaction carried out a constant temperature and pressure.
IF ΔG<0, the reaction is spontaneous.
If ΔG>0, the reaction is non-spontaneous.
If ΔG=0, the reaction is at equilibrium.
Read the above paragraph carefully and answer the following questions based on the above comprehension.
If an endothermic reaction is non-spontaneous at freezing point of water and becomes feasible at its boiling point, then
View SolutionThe ellingham diagram for zinc, magnesium and carbon coverting into correspondingoxides is shown below.
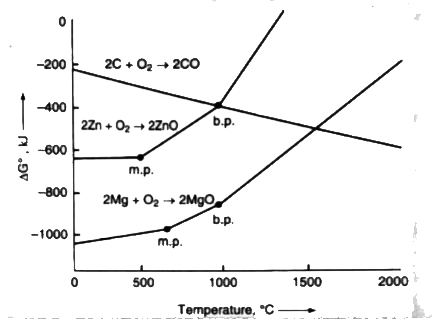
At 1100∘C, which reaction is spontaneous to a maximum extent ?
View SolutionThe ellingham diagram for zinc, magnesium and carbon coverting into correspondingoxides is shown below.

At 1100∘C, which reaction is spontaneous to a maximum extent ?
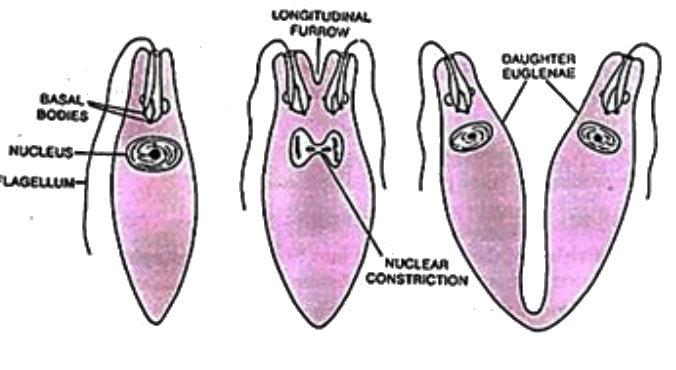
View SolutionThe given below diagram is the representation of
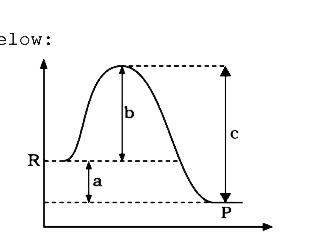
View SolutionThe potential diagram for reaction R→P is given below:
ΔH∘ of the reaction corresponds to the energy
View Solution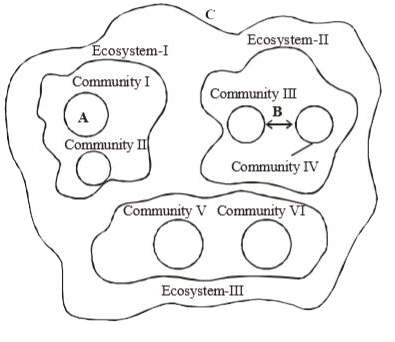
In the given diagram which diversity represent by A, B, and C
View Solution

