Read the given statements and select the correct option.
Statement 1: A salt is produced when an acid is neutralised by a base. Statement 2: A salt can be acidic, basic or neutral.
Both statements 1 and 2 are true and statement 2 is the correct explanation of statement 1.
Both statements 1 and 2 are true but statement 2 is not the correct explanation of statement 1.
Statement 1 is true and statement 2 is false.
Both statements 1 and 2 are false.
The correct Answer is:B
To solve the question, we need to analyze both statements provided and determine their validity.
Step 1: Analyze Statement 1
Statement 1: A salt is produced when an acid is neutralised by a base.
- When an acid reacts with a base, a chemical reaction occurs that typically produces a salt and water. For example, when hydrochloric acid (HCl) reacts with sodium hydroxide (NaOH), the products are sodium chloride (NaCl), which is a salt, and water (H2O).
- Therefore, Statement 1 is true.
Step 2: Analyze Statement 2
Statement 2: A salt can be acidic, basic or neutral.
- Salts can indeed be classified based on the strength of the acid and base from which they are formed:
- Acidic salts are formed from a strong acid and a weak base (e.g., ammonium chloride, NH4Cl).
- Basic salts are formed from a weak acid and a strong base (e.g., sodium bicarbonate, NaHCO3).
- Neutral salts are formed from a strong acid and a strong base (e.g., sodium chloride, NaCl).
- Therefore, Statement 2 is also true.
Conclusion
Both statements are true, but the question asks for the correct option based on the analysis of these statements.
Final Answer
Both statements are correct, so the correct option is Option 2.
---
Statement 1: A salt is produced when an acid is neutralised by a base.
- Therefore, Statement 1 is true.
Statement 2: A salt can be acidic, basic or neutral.
- Acidic salts are formed from a strong acid and a weak base (e.g., ammonium chloride, NH4Cl).
- Basic salts are formed from a weak acid and a strong base (e.g., sodium bicarbonate, NaHCO3).
- Neutral salts are formed from a strong acid and a strong base (e.g., sodium chloride, NaCl).
- Therefore, Statement 2 is also true.
Both statements are true, but the question asks for the correct option based on the analysis of these statements.
Both statements are correct, so the correct option is Option 2.
Topper's Solved these Questions
- NSO QUESTION PAPER 2018-19 SET ABOOK - SCIENCE OLYMPIAD FOUNDATION CHAPTER - NSO QUESTION PAPER 2018-19 SET AEXERCISE - ACHIEVERS SECTION
1 Video
- NSO QUESTION PAPER 2018- 19 SET - BBOOK - SCIENCE OLYMPIAD FOUNDATION CHAPTER - NSO QUESTION PAPER 2018- 19 SET - BEXERCISE - ACHIEVERS SECTION
1 Video
- NSO QUESTION PAPER 2019- 20 SET - BBOOK - SCIENCE OLYMPIAD FOUNDATION CHAPTER - NSO QUESTION PAPER 2019- 20 SET - BEXERCISE - ACHIEVERS SECTION
1 Video
Similar Questions
Leena had set up the experiment as shown in the figure.
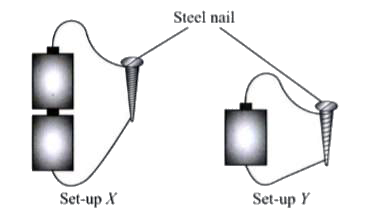
She found out that the steel nail in set-up X had attracted more iron pins than the steel nail in set-up Y. What could be the reason for this?
I. The batteries used in set-up X are more expensive. There are more batteries used in set-up X. III. The number of turns of coil around the nail in set-up Y is fewer than in set-up X. IV. A longer wire was used in set-up X.
View SolutionObserve the given figures carefully.
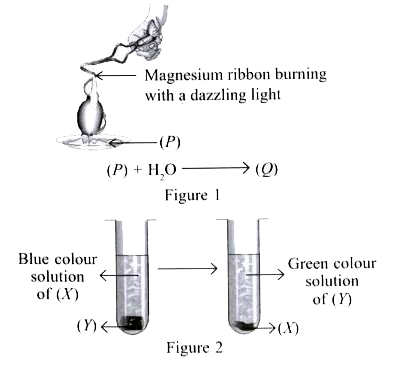
Read the given passage and fill in the blanks by selecting an appropriate option.
Figures 1 and 2 both represent a (i)___ change. In figure 1, P is (ii)___ and Q is (iii)___. In figure 2, X and Y can be (iv)___ and (v) ____ respectively.
View SolutionRead the given statements.
(a) During______, the sheared skin of sheep with hair is washed to remove grease, dust and dirt. (b)_____ is an occupational disease associated with wool industry. ( c) The clean and selected wool fibres are passed through rollers having thin wired teeth that untangle the fibres and arrange them into a flat sheet called _______ (d) Pashmina shawls are made from wool obtained from the undercoat of_____.
Select the option which correctly fills the blanks in any two of these statements.
View SolutionStatement-1: NaCl cannot be hydrolysed.
Statement-2: It is a salt of strong acid and strong base.
View SolutionStatement-1: Aqueous solution of ammonium carbonate is basic. Statement-2: Acidic/basic nature of a salt solution of a salt of weak acid and weak base depends on K_(a) and K_(b) value of the acid and the base forming it.
View SolutionCertain statements which describe different types of salts are given below. (a) Two basic and one acidic radicals are present in one molecule of this salt. (b) This salt reacts further with a base. (c) This salt consists of one basic radical and one acidic radical and does not react further either with an acid or with a base. (d)This salt is a combination of two simple salts. (e) This salt reacts further with an acid. Arrange the statements in the following order. {:((i),"Acidic salt",(ii),"Basic salt"),((iii),"Normal salt",(iv),"Double salt"),((v),"Mixed salt",,):}
View SolutionSTATEMENT - 1 : solubility of sulphide is higher in acidic medium than pure water. STATEMENT - 2 : Aq. Solution of metal sulphides is neutral. STATEMENT - 3 : Metal sulphides are salt of strong base and weak acid.
View Solutionकथन-1-प्रबल अम्ल और प्रबल क्षार के उदासीनीकरण की एन्थैल्पी स्थिरांक होती है।
कथन-2-अम्ल और भस्म की अभिक्रिया से लवण और जल बनते हैं।
View Solutionಪ್ರಬಲ ಆಮ್ಲ ಮತ್ತು ಪ್ರಬಲ ಪ್ರತ್ಯಾಮ್ಲಗಳ ಲವಣಗಳು ತಟಸ್ಥವಾಗಿದ್ದು ಅವುಗಳ ಮೌಲ್ಯ :
View SolutionChemical formulas|Acids|Bases|Salts|Neutralisation
View SolutionThe salt solution NH4OH and HCl is acidic basic or neutral ?
View Solutionवक्तव्य I अमोनियम काबोंनेट का जलीय विलयन क्षारकीय होता है।
वक्तव्य II दुर्बल अम्ल तथा दुर्बल क्षारक के लवण के विलयन की अम्लता। क्षारकीयता इसे बनाने वाले अम्ल तथा क्षारक के क्रमश: Ka तथा Kb मान पर निर्भर करती है।
View SolutionWhich statement is wrong ? 1) Acid + Metal → Salt +H2. 2) Base + Metal→ Salt + CO2. 3) Metal Carbonate + Acid → Salt + CO2 +Water. 4) Metal hydrogen carbonate + Acid → Salt + H2 + Water
View SolutionStatement A : substances whose solutions are neutral are salts. Statement B : Salts need not always be neutral, they can be acidic or basic.
View SolutionWhat are neutral salts, acidic salts and basic salts. Give two examples each of neutral, acidic and basic salts.
View SolutionSTATEMENT - 1 : solubility of sulphide is higher in acidic medium than pure water. STATEMENT - 2 : Aq. Solution of metal sulphides is neutral. STATEMENT - 3 : Metal sulphides are salt of strong base and weak acid.
View Solution

