Rules for to decide relative stability for different resonance structure
Text Solution
(i) Among the resonance structures, the one which has more number of covalent bonds, all the atoms with octet of electrons (except hydrogen which has a duplet) are more stable. (ii) Among the resonance structures, less separation of opposite charges, (a negative charge if any on more electronegative atom, a positive charge if any on more electropositive atom) and more dispersal of charge, is more stable than others.
|
Similar Questions
Knowledge Check
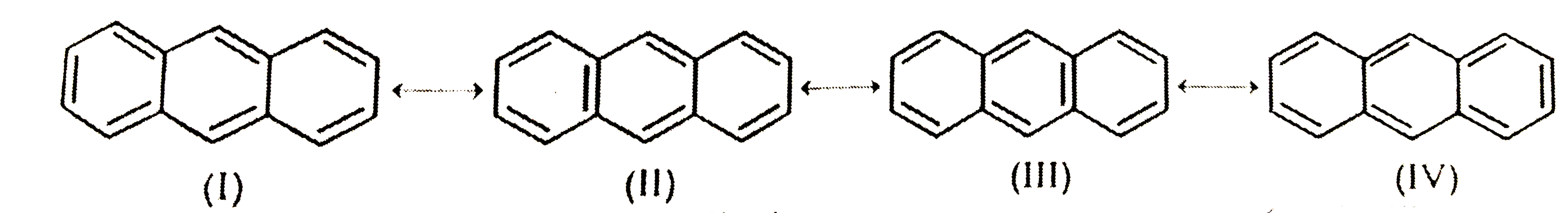
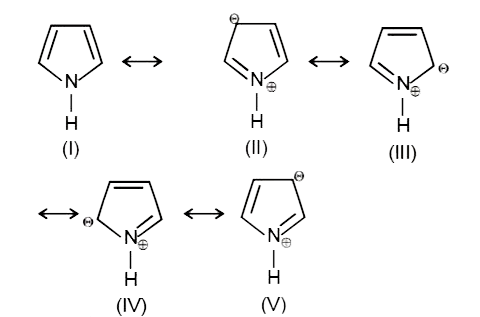
The correct stability order of given resonating structures
 AI>II>III>IVBIV>III>II>ICI=II=III=IVDII=III>I=IV
AI>II>III>IVBIV>III>II>ICI=II=III=IVDII=III>I=IVCompare relative stability of the following resonating structure.
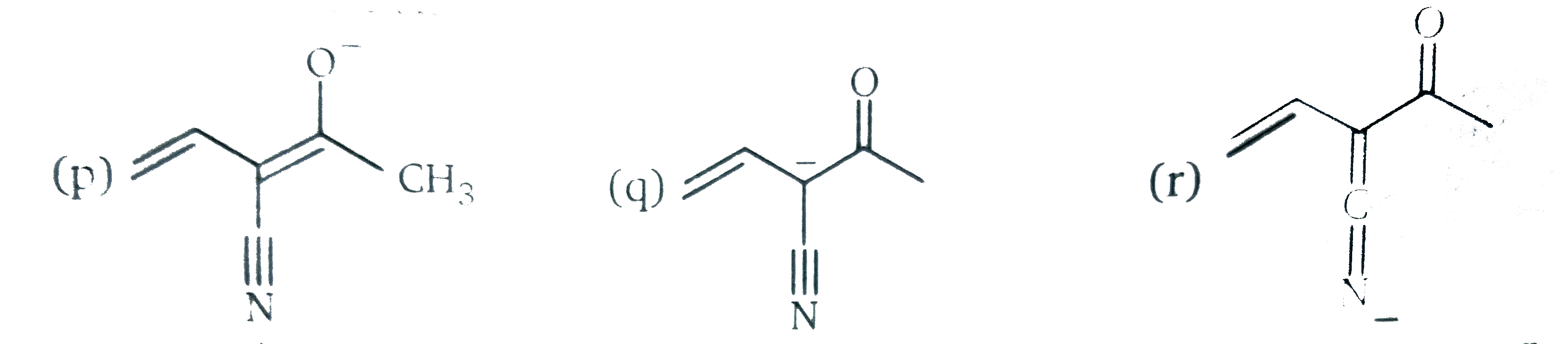 Ap>q>rBq>p>rCq>r>pDp>r>q
Ap>q>rBq>p>rCq>r>pDp>r>qThe correct stability order of the following resonance structure is
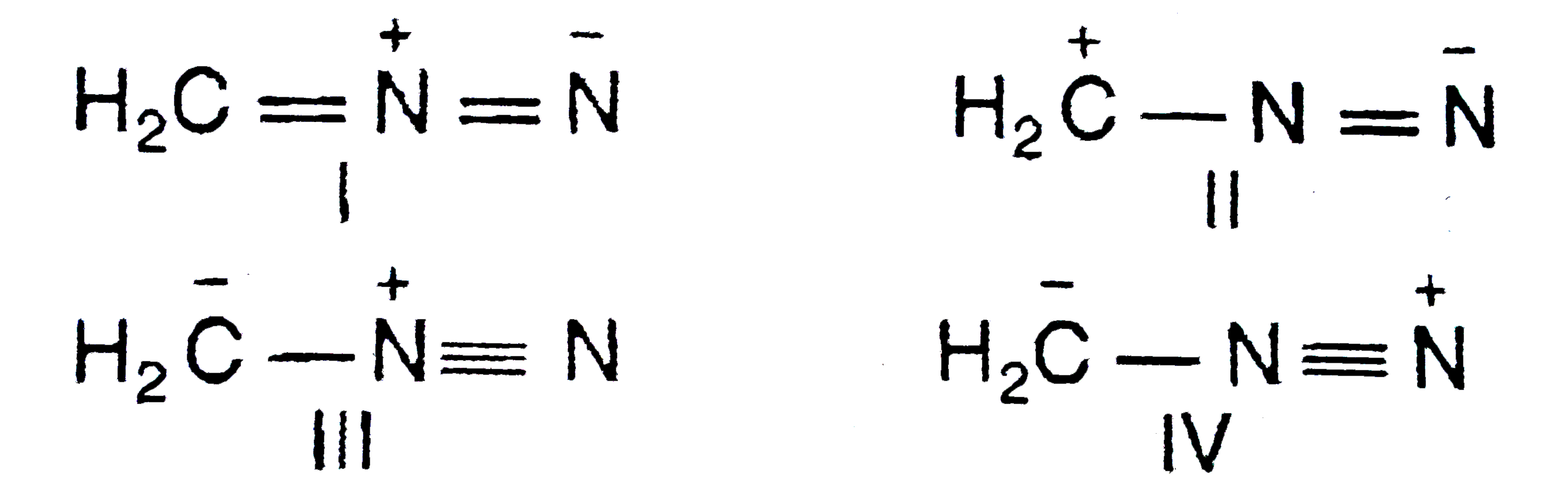 AI>II>IV>IIIBI>III>II>IVCII>I>III>IVDIII>I>IV>II
AI>II>IV>IIIBI>III>II>IVCII>I>III>IVDIII>I>IV>II
Write the stability order of following resonating structures :
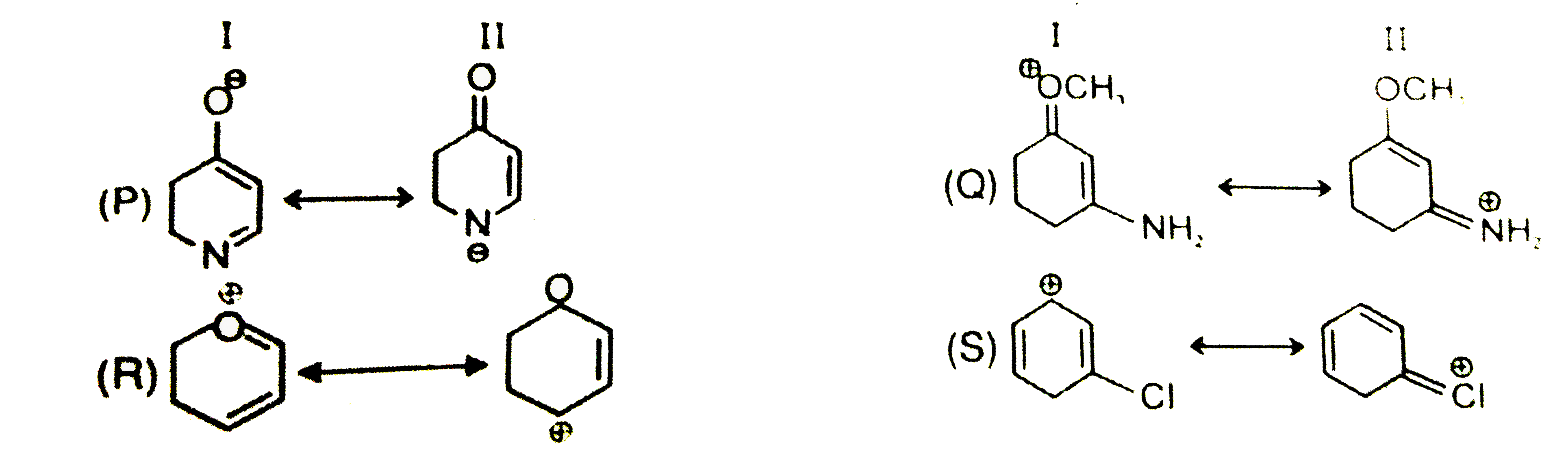
View SolutionComparison OF Stabilities OF Different Resonating Structures
View Solution|| Stability and comparison OF resonating structure ||
View SolutionWrite the stability order of Resonating Structures:
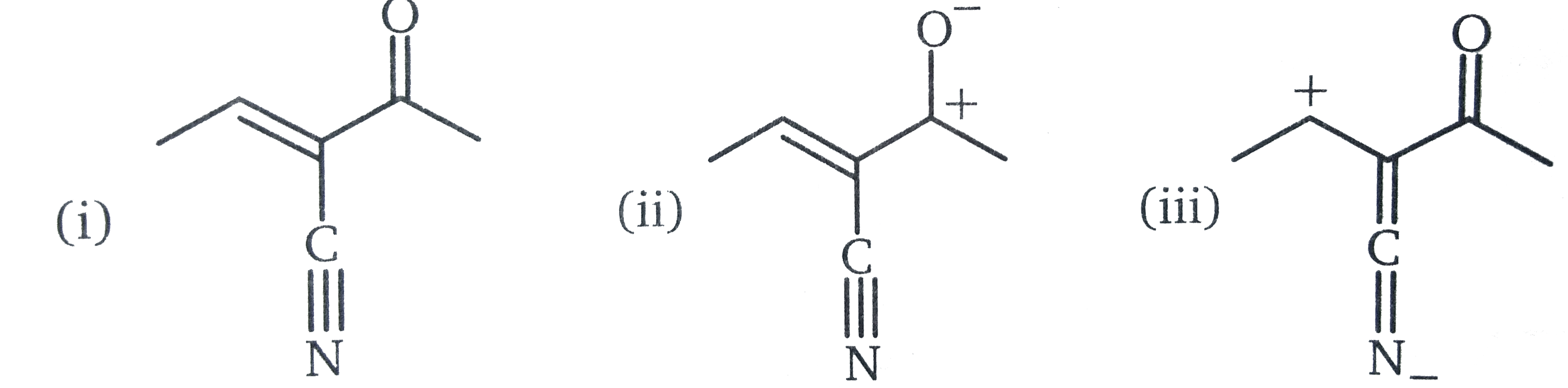
View SolutionCompare relative stability of the following resonating strucrtrue.
View Solution