Spectral line given by an atom is a kind of signature of the respective atom. Commnet of this statement.
(i) calcualtion of energy of electrons in He+ and Li+2 ions.
(ii) 4:9
Similar Questions
Knowledge Check
Number of spectral lines in hydrogen atom is
A3B6C15DInfiniteThe shortest wavelength spectral line in the brackett series of the spectrum of atomic hydrogen is the transition :
An2=∞→n1=1Bn2=8→n1=4Cn2=∞→n1=4Dn2=4→n1=3
Spectral Line Calculation of H-atom and Question Discussion
View SolutionThe shortest wavelength spectral line in the brackett series of the spectrum of atomic hydrogen is the transition :
View SolutionAt some place in universe an atom consists of a positron revolving round an antiproton. The ratio of the wavelength of the corresponding spectral lines from this atom and ordinary hydrogen atoms is
View SolutionCalculate the maximum no. of spectral lines obtained if 4-H-atoms are present, if an electron jumps from 4th excited state to ground state in H atom.
View SolutionGiven below are the spectral lines for an atom of hydrogen. Mark the lines which are not correctly matched with the value of n1 and n2?
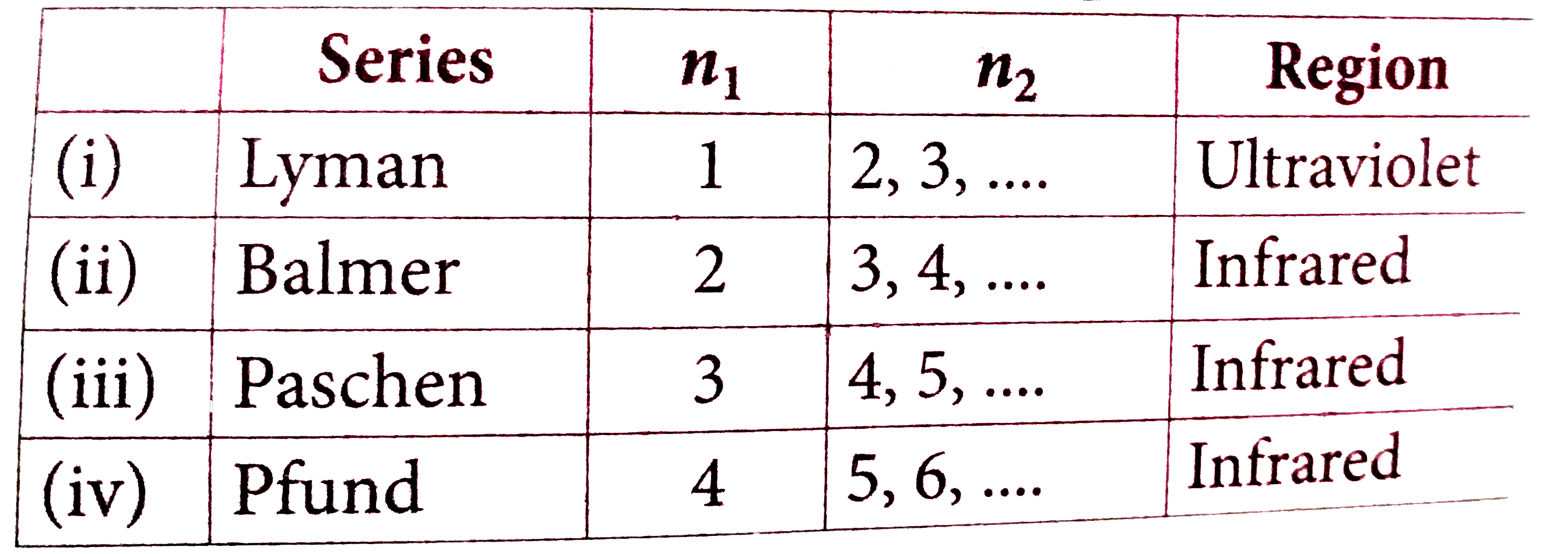
View SolutionGiven below are the spectral lines for an atom of hydrogen. Mark the lines which are not correctly matched with the value of n1 and n2?
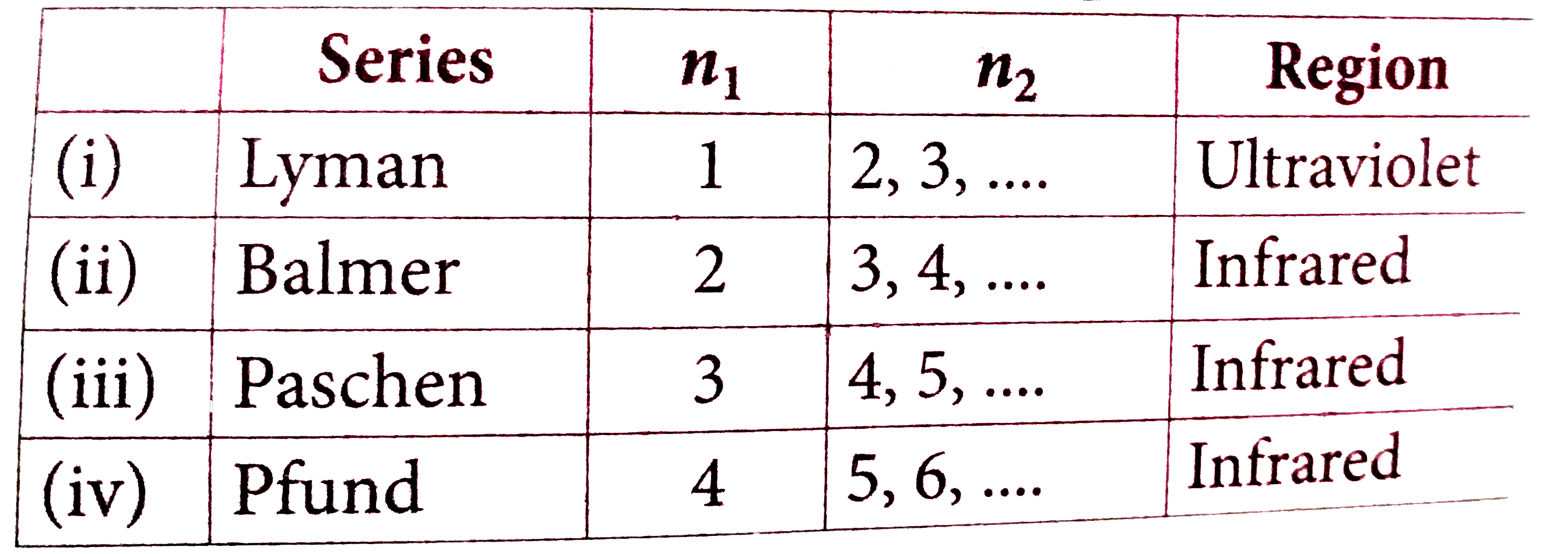
View Solution
