The 1st,2nd and the 3rd ionization enthalpies. I1,I2,I3 of four atoms with atomic numbers n,n+1,n+2andn+3, where n<10 , are tabulated below. What is the value of n?
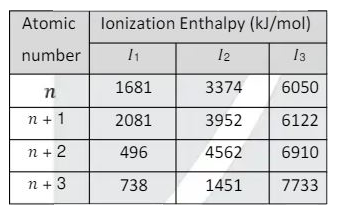
The correct Answer is:9
From given data (n + 2) atom is alkali metal which is sodium.
As n+2=11 so (n = 9)
Similar Questions
Knowledge Check
What is the modulus of the complex number i2n+1(−i)2n−1, where n∈N and i=√−1?
A−1B1C√2D2If i=√−1 and n is a positive integer, then in+in+1+in+2+in+3
A1BiCinD0The value of i2n+i2n+1+i2n+2+i2n+3, where i=√−1 is :
A0B1CiD−i
Consider the following three compounds (i)AXn−2n, (ii)AX3n and (ii)AXn+4n, where central atom A is 15th group element and their maximum covalency is 3n. If total number of proton in surrounding atom X is n and value of n is one, then calculate value of x3+y2+z2 . (where x, y and z are total number of lone pair at central atom in compounds (i), (ii) and (iii) respectively.
View SolutionThe set (0,2,6,12,20) in the set-builder form is
(1){x:x=n2−3n+2, where n' is a natural number &1≤n≤5}
(2){x:x=n2−3n+2, where 'n' is a natural number &1≤n≤6}
(3){x:x=n2−3n+4, where 'n' is a natural number &1≤n≤5}
(4){x:x=n2+5n−6, where 'n' is a natural number &1≤n≤5}
View SolutionConsider the following three compounds (i)AXn−2n, (ii)AX3n and (ii)AXn+4n, where central atom A is 15th group element and their maximum covalency is 3n. If total number of proton in surrounding atom X is n and value of n is one, then calculate value of x3+y2+z2 . (where x, y and z are total number of lone pair at central atom in compounds (i), (ii) and (iii) respectively.
View SolutionConsider the following three compounds (i)AXn−2n, (ii)AX3n and (ii)AXn+4n, where central atom A is 15th group element and their maximum covalency is 3n. If total number of proton in surrounding atom X is n and value of n is one, then calculate value of x3+y2+z2 . (where x, y and z are total number of lone pair at central atom in compounds (i), (ii) and (iii) respectively.
View SolutionWhat is the value of the sum 11∑n=2(in+in+1), where i=√−1 ?
View Solution
