The charges on two elements A and B are given below:

what is the formula of sulphate of A?
Formula of sulphate of A
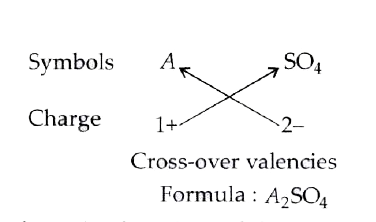
Thus, the formula of sulphate of element A is A2SO4.
Topper's Solved these Questions
- MATTER : ELEMENTS COMPOUNDS AND MIXTURES Book:MTG IIT JEE FOUNDATIONChapter:MATTER : ELEMENTS COMPOUNDS AND MIXTURES Exercise:EXERCISE (MULTIPLE CHOICE QUESTIONS)
Explore 50 Videos
- MATTER : ELEMENTS COMPOUNDS AND MIXTURES Book:MTG IIT JEE FOUNDATIONChapter:MATTER : ELEMENTS COMPOUNDS AND MIXTURES Exercise:EXERCISE (MATCH THE FOLLOWING)
Explore 10 Videos
- MATTER : ELEMENTS COMPOUNDS AND MIXTURES Book:MTG IIT JEE FOUNDATIONChapter:MATTER : ELEMENTS COMPOUNDS AND MIXTURES Exercise:EXERCISE (INTEGER/NUMERICAL VALUE )
Explore 5 Videos
- MATERIALS : METALS AND NON-METALSBook:MTG IIT JEE FOUNDATIONChapter:MATERIALS : METALS AND NON-METALSExercise:Olympiad/HOTS Corner
Explore 20 Videos
Similar Questions
Knowledge Check
The formula of barium sulphate is
ABa2SO4BBaSO4CBa(SO4)2DBa2(SO4)3Formula of ferric sulphate is
AFeSO4BFe(SO4)2CFe2SO4DFe2(SO4)3Ionization energies of five elements in kcal/mol are given below:
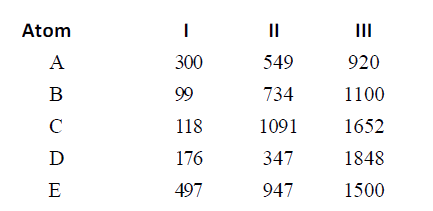 .
.
If B react with fluorine and oxygen, the molecular formula of fluoride and oxide will be respectively :ABF3,B2O3BBF,B2OCBF2,BODnone of these
Work out the formula for aluminium sulphate .
View SolutionIonization energies of five elements in kcal/mol are given below:
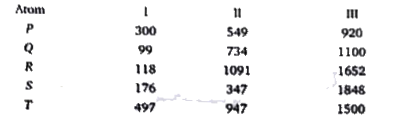
Q. If Q reacts with fluorine and oxyge, the molecular formula of fluoride and oxide will be respectively:
View SolutionThe unit cell of a compound made up of the three elements X, Y and Z is given below.
 The formula of this compound is
The formula of this compound is
View SolutionIonization energies of five elements in kcal/mol are given below:
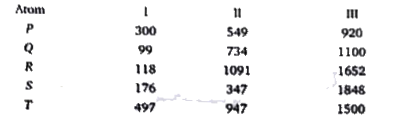
Q. If Q reacts with fluorine and oxyge, the molecular formula of fluoride and oxide will be respectively:
View SolutionIonization energies of five elements in kcal/mol are given below :
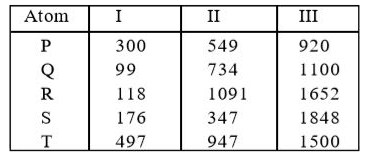
If Q reacts with fluorine and oxygen, the molecular formula of fluoride and oxide will be respectively :-
View Solution