The given graph shows the vapour pressure - temperature curves for some liquids
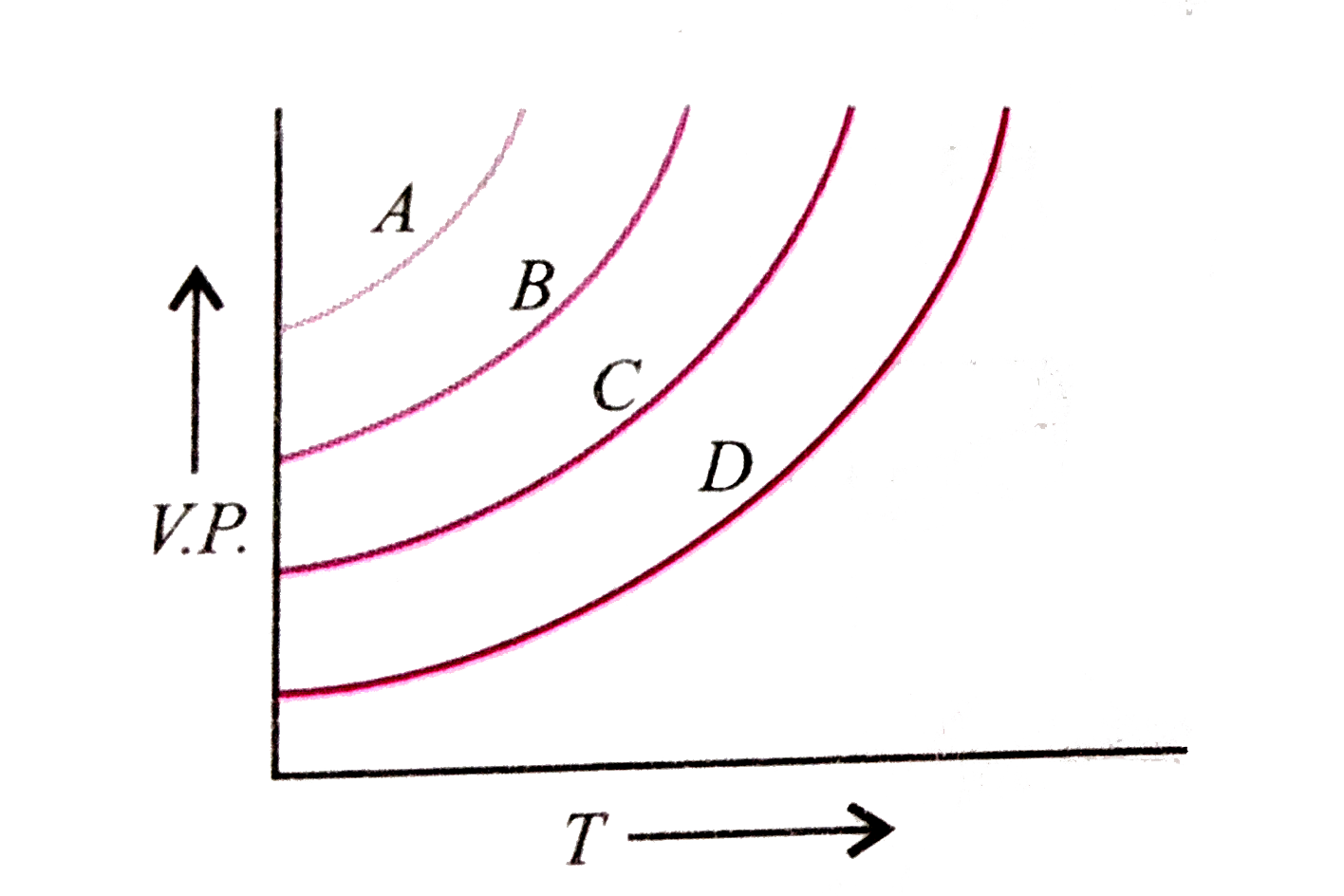
Liquids A,B, C and D respectively are
diethyl ether, acetone , ethyl alcohol , water
acetone , ethyl alcohol, diethyl ether , water
water, ethyl alcohol , acetone , diethyl ether
ethyl alcohol, acetone , diethyl ether, water
The correct Answer is:A
The vapour pressure increases with decrease in intermolecular forces . When the forces are weak, the liquid has high volatility and maximum vapour pressure . Diethyl ether has highest vapour pressure while water has lowest vapour pressure.
Topper's Solved these Questions
Similar Questions
Knowledge Check
The given graph shows the vapour pressure temperature curves for some liquids.
Liquids A, B, C and Drespectively are,AEthyl alcohol, acetone, diethyl ether, waterBWater, ethyl alcohol, acetone, diethyl etherCAcetone, ethyl alcohol, diethyl ether, waterDDiethyl ether, acetone, ethyl alcohol, waterVapour pressure of a liquid decreases with
Adecrease in temperatureBincrease in temperatureCincrease in surface areaDincrease in volume .
A graph between vapour pressure and temperature of few liquids is given below . Study the graph and answer the following :
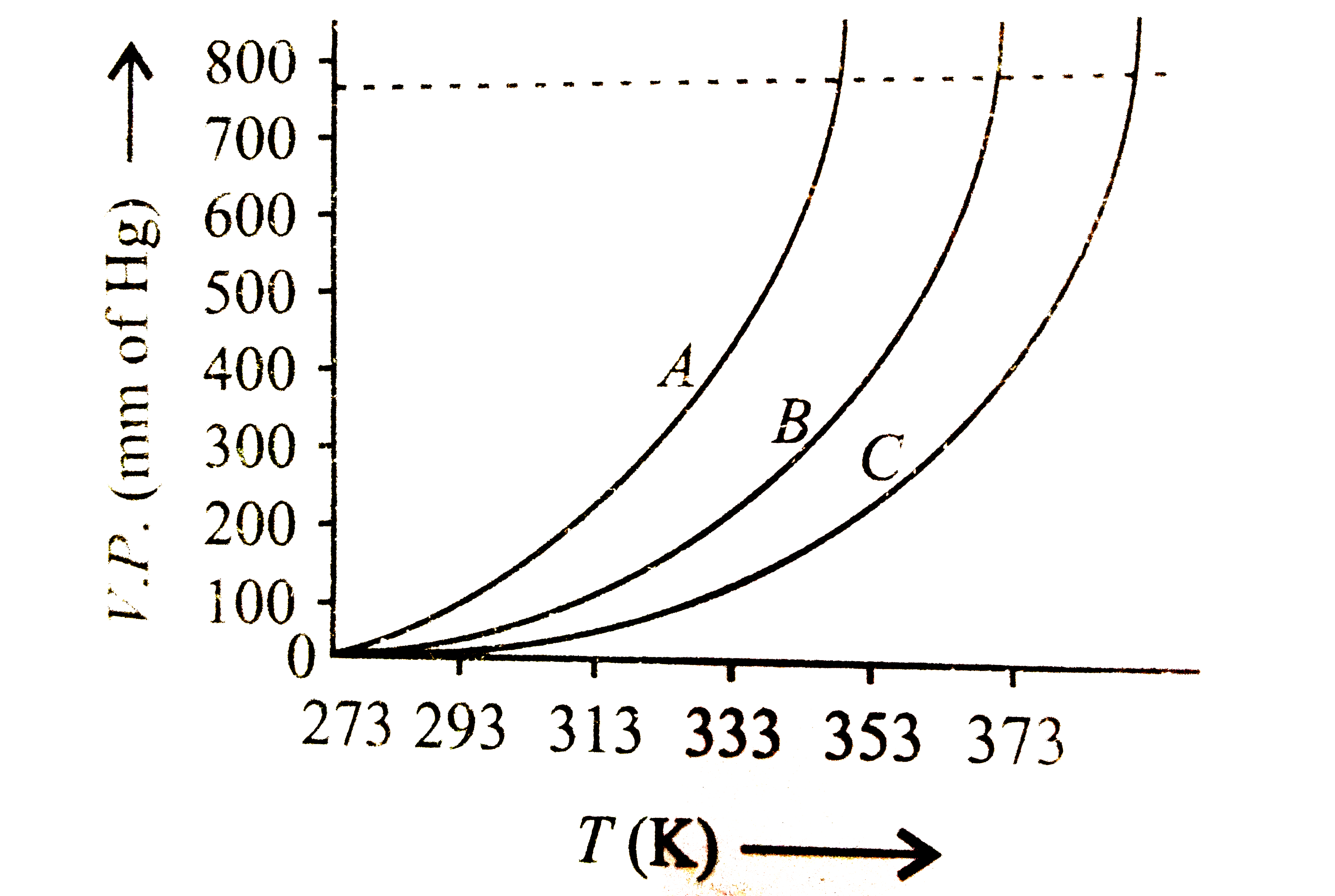
Which of the following statements is true ?
View SolutionVapour pressure of a pure liquid does not depend upon
View SolutionThe vapour pressure of a given liquid will increase if
View SolutionIdentify which liquid will have a higher vapour pressure at 90∘C If the boiling point of two liquids A and B are 140∘ C and 180∘ C, respectively.
View SolutionThe following is a graph plotted between the vapour pressure of two volatile liquids against their respective mole fractions. Which of the following statements is/are correct?
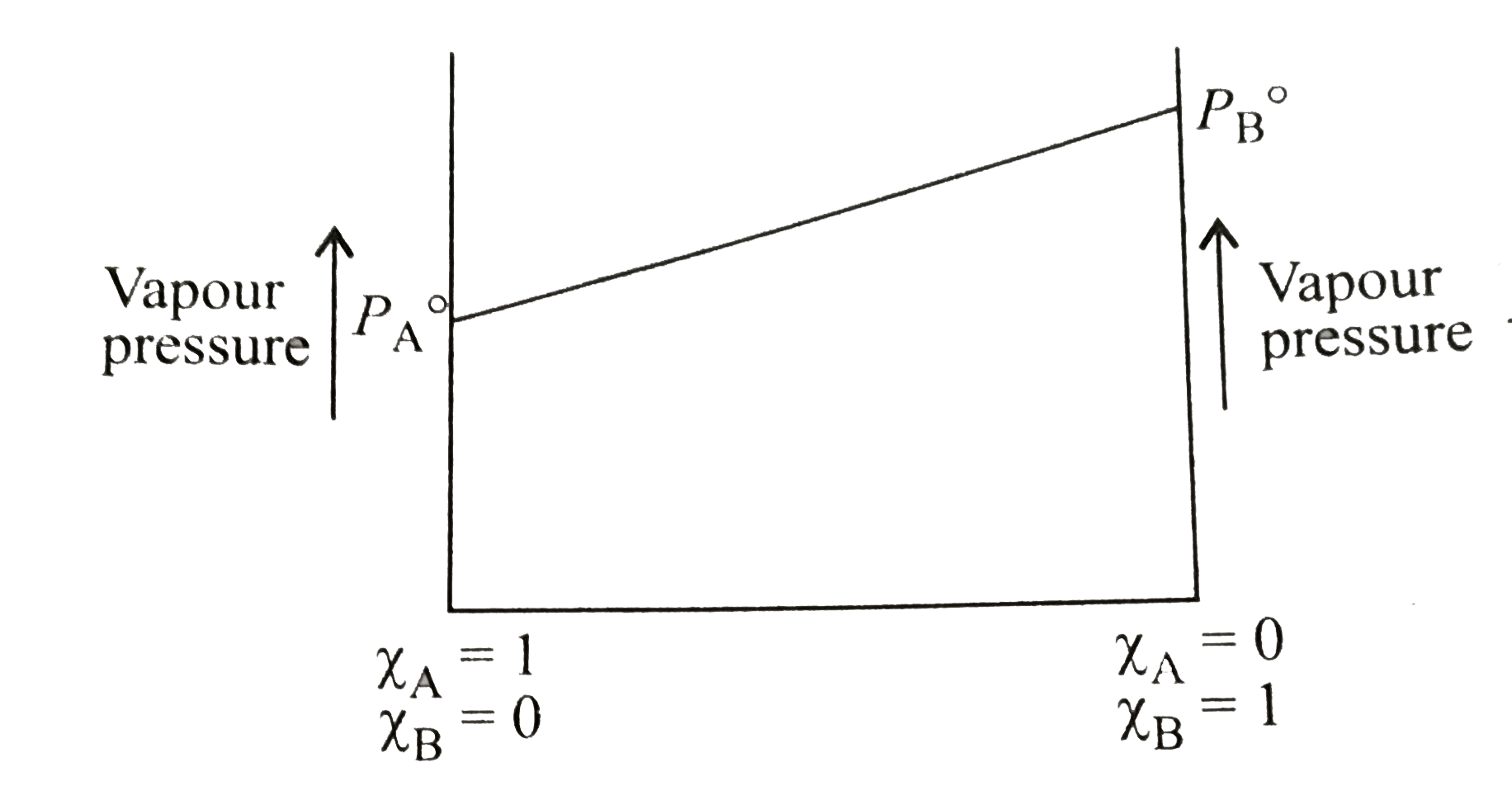
View SolutionThe vapour pressure of a given liquid will decrease if :
View Solution

