The number of electrons in an element with atomic number X and mass number Y will be
X-Y
Y-X
X+Y
X
The correct Answer is:D
X
Topper's Solved these Questions
- ATOMIC STRUCTUREBook:S CHAND IIT JEE FOUNDATIONChapter:ATOMIC STRUCTUREExercise:QUESTION BANK-20 ( Answer the following questions )
Explore 4 Videos
- ATOMIC STRUCTUREBook:S CHAND IIT JEE FOUNDATIONChapter:ATOMIC STRUCTUREExercise:SELF ASSESSMENT SHEET -20
Explore 23 Videos
- ATOMIC STRUCTUREBook:S CHAND IIT JEE FOUNDATIONChapter:ATOMIC STRUCTUREExercise:QUESTION BANK-20 (FILL IN THE BLANKS WITH APPROPRIATE WORDS TO MAKE CORRECT STATEMENTS. )
Explore 10 Videos
- CARBON AND ITS COMPOUNDS Book:S CHAND IIT JEE FOUNDATIONChapter:CARBON AND ITS COMPOUNDS Exercise:SELF ASSESSMENT SHEET-19
Explore 24 Videos
Similar Questions
What particle is needed to begin a nuclear chain reaction?
View SolutionWhat is the maximum number of electrons which can be accomodated in the L shell of an atom ?
View SolutionThe electrons present in the outermost shell of an atom are called_____ electrons.
View SolutionMatch correctly
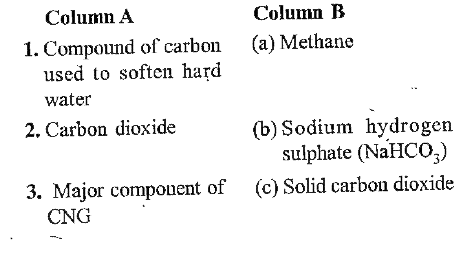
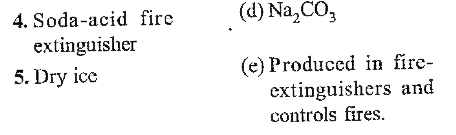
View SolutionThe mass number of an element is twice its atomic number. If there are four electrons in 2p orbitals, Write the electronic configuration of the element and name it.
View SolutionThe atomic number of an element Y is 17. The number of electrons in its ion Y^(-) will be:
View SolutionIf the atomic number (Z) and mass number (A) of an element X are related by the equation A+Z= 46 and the total number of neutrons in one atom of X is 16, then the total number of protons and electrons in one atom of element X='a'. Find value of 'a'
View Solutionकिसी तत्व की द्रव्यमान संख्या 32 तथा इसका परमाणु क्रमांक 16 है । तत्व में इलेक्ट्रॉनों की संख्या क्या होगी ?
View SolutionElectronic configuration of the element with atomic number 56 and mass number 138 is
View SolutionThe number of electrons in an element with atomic number X and mass number Y will be
View SolutionElectronic configuration of the element with atomic number 56 and mass number 138 is
View SolutionElectronic configuration of the element with atomic number 56 and mass number 138 is
View SolutionFind out atomic number, mass number, number of electron and neutron in an element? X(Atomic no 20, Mass no. 40)
View Solutionयदि एक तत्वा X की परमाणु संख्या (Z) व द्रवमान संख्या (A) , समीकरण A+Z=46 के अनुसार संबन्धि है तथा X के एक परमाणु में न्यूट्रॉनों की कुल संख्या 16 है, तब X तत्व के एक परमाणु में प्रोटॉन तथा इलेक्ट्रॉन की कुल संख्या = .a.. .a. का मान ज्ञात कीजिए।
View SolutionIf the atomic number (Z) and mass number (A) of an element X are related by the equation A+Z= 46 and the total number of neutrons in one atom of X is 16, then the total number of protons and electrons in one atom of element X='a'. Find value of 'a'
View SolutionThe mass number of an element is 23 and the atomic number is 11. What is the number of electrons?
View Solution